Cryo-EM visualization of membrane damage by bacterial toxin
Pore forming toxins (PFTs) are virulence factors which assist pathogens in colonising host organisms. With the growing risk of multidrug resistance (MDR) in microorganisms, intensive study of PFTs has garnered worldwide attention. PFTs do not just puncture holes and make cells more permeable, many of them also hijack housekeeping functions of the host and lead to various pathophysiological conditions such as Toxic Shock Syndrome, diphtheria, anthrax or cholera. The Vibrio cholerae cytolysin (VCC) from the pathogenic bacterium Vibrio cholerae is an archetypical example of a PFT.
A recent study led by Somnath Dutta, Assistant Professor in the Molecular Biophysics Unit (MBU), sheds light on the probable pore formation mechanism of VCC. Dutta’s team used cutting-edge cryo-electron microscopy and sophisticated image processing techniques to show the miniscule VCC toxin embedded in a real lipid membrane environment. Dr Kausik Chattopadhyay, Professor at IISER Mohali, was a collaborator in this work. This novel approach of studying a PFT in a native environment revealed various structural intermediates of VCC, each of which had different levels of lipid engagement. These intermediate conformations could help scientists understand the stages of membrane permeabilization. With the high-resolution structure of lipid-bound VCC, the researchers were able to identify primary amino acid residues which interact with the membrane and lay a robust foundation for the pore formation.
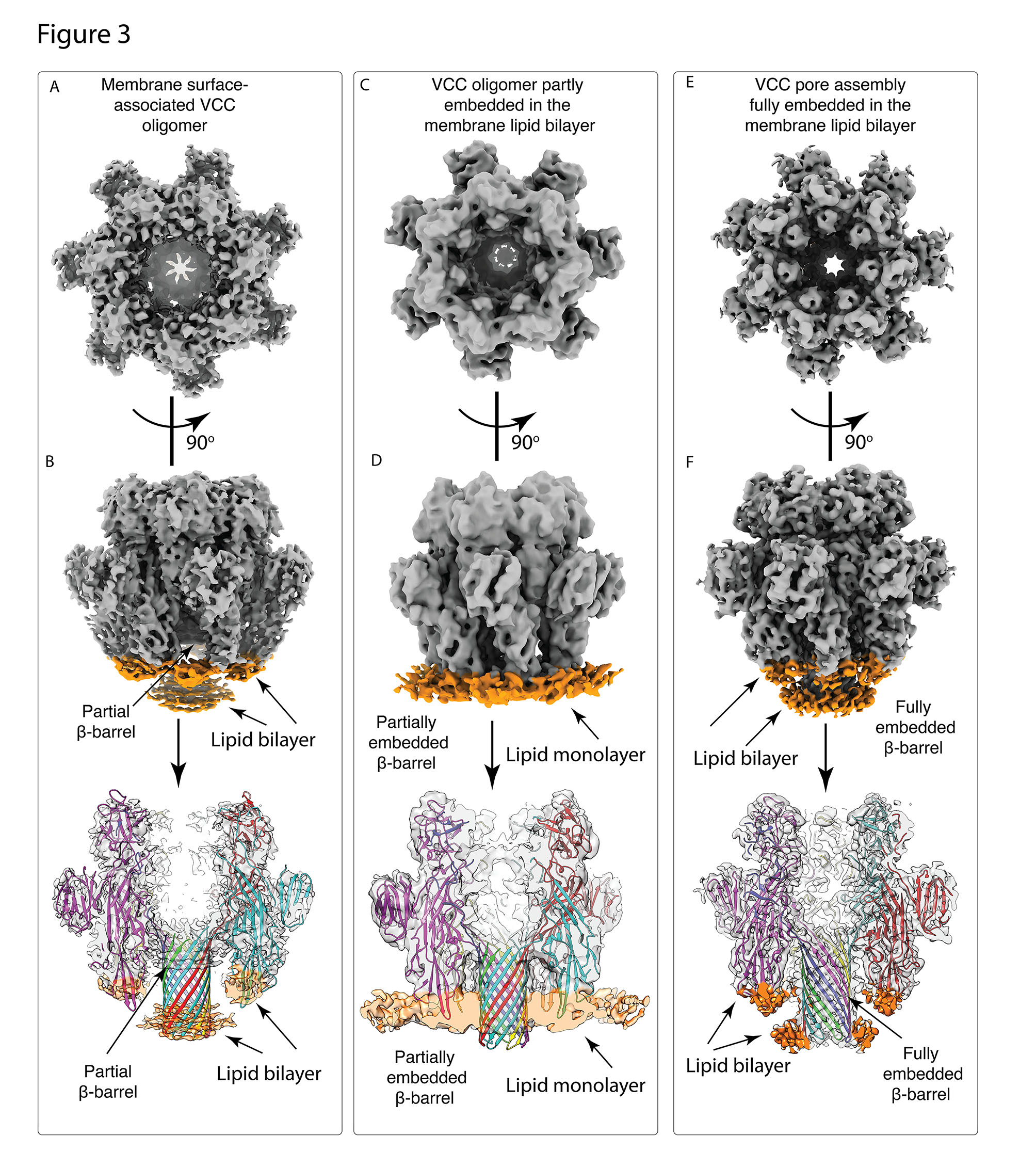
Comparison of the three distinct structural states of VCC in a lipid bilayer environment: (A)-(B) VCC structure which is adhered to the surface of the membrane (C)-(D) VCC structure which is partially embedded within membrane (E)-(F) VCC structure which is completely embedded within the membrane.
Figure adapted from Sengupta et al, 2021. https://doi.org/10.1083/jcb.202102035
The novel insights from this study will provide a new outlook on other PFTs of the same family, such as all Staphylococcal toxins and the Anthrax toxin, which share a common pore architecture. Understanding the structural basis for membrane lysis may potentially help in the development of alternative therapeutic targets against this large repertoire of virulent microbes.
REFERENCE:
Nayanika Sengupta, Anish Kumar Mondal, Suman Mishra, Kausik Chattopadhyay, Somnath Dutta, Single-particle cryo-EM reveals conformational variability of the oligomeric VCC β-barrel pore in a lipid bilayer, Journal of Cell Biology (2021).
https://doi.org/10.1083/jcb.202102035
Funding: This work is supported by Department of Biotechnology (DBT), Department of Science and Technology (DST-FIST). The authors would like to acknowledge the National Cryo-EM Facility, IISc for cryo-EM data collection.
LAB WEBSITE:
https://somnath92.wixsite.com/website
http://mbu.iisc.ac.in/SDLab.htm/






