Fate of an Intruder in Colloidal Nanocrystals is Governed by Entropy
 The physics of strongly confined systems of interacting particles has been a subject of active research for more than a century. These are systems consisting of a small number of particles confined in a small region by an external potential or small clusters formed due to inherent inter-particle interactions. Such systems are ubiquitous in nature – examples include petroleum in porous rocks, cytoplasm in biological cells and electrons in a quantum dot. Physical properties of such systems are substantially different from those of macroscopic (bulk) systems because the fraction of particles on the surface of a small cluster is larger than that for a bulk system and this fraction increases as the size of the cluster is reduced. Also, small local changes inside a cluster, such as the presence of a single impurity or the formation of a single defect, can affect the properties of the whole cluster if it is small. For these reasons, physical processes such as crystallization, glass formation and defect dynamics in strongly confined systems can exhibit characteristics that are fundamentally different from those found in their bulk counterparts. The development of an understanding of these effects of strong confinement is of fundamental importance in the design and assembly of nanomaterials.
The physics of strongly confined systems of interacting particles has been a subject of active research for more than a century. These are systems consisting of a small number of particles confined in a small region by an external potential or small clusters formed due to inherent inter-particle interactions. Such systems are ubiquitous in nature – examples include petroleum in porous rocks, cytoplasm in biological cells and electrons in a quantum dot. Physical properties of such systems are substantially different from those of macroscopic (bulk) systems because the fraction of particles on the surface of a small cluster is larger than that for a bulk system and this fraction increases as the size of the cluster is reduced. Also, small local changes inside a cluster, such as the presence of a single impurity or the formation of a single defect, can affect the properties of the whole cluster if it is small. For these reasons, physical processes such as crystallization, glass formation and defect dynamics in strongly confined systems can exhibit characteristics that are fundamentally different from those found in their bulk counterparts. The development of an understanding of these effects of strong confinement is of fundamental importance in the design and assembly of nanomaterials.
In our work, we have addressed the following key question: if a dopant (impurity) is placed inside a strongly confined colloidal nanocrystal, then how does the combined system of dopant and crystallite evole in time? Is the dopant incorporated permanently in the crystallite or expelled spontaneously?
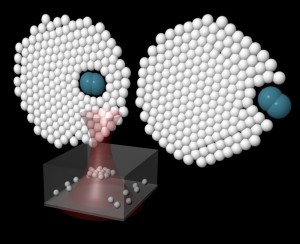 Our experimental system consists of a collection of colloidal particles and a dopant in a highly configurable confining potential, formed using a defocussed optical tweezer. By varying the power and level of defocussing, colloidal assemblies of different sizes and phases (liquid-like or crystalline) could be formed. Most importantly, we have studied the dynamics of foreign dopants, in the form of particles of different shapes and sizes, injected into the crystallites. The striking result obtained here was the ability of finite-sized colloidal clusters to expel or internalize a foreign dopant depending on its initial position. This surprising phenomenon could be reproduced in our simulations which also provided an explanation of this behaviour. We found that the fate of a dopant is governed to a large extent by the entropy of the system, which becomes increasingly important as the size of the crystallite is reduced. The internal energy of the system was found to decrease as the dopant gets expelled, which by itself would imply expulsion of the dopant for all initial positions. However, the entropy of the system also decreases as the dopant is expelled, because the system gets more ordered in this process. This effect favours internalization of the dopant. Careful observations show that the rate of change of the entropy with increasing distance of the dopant from the centre of the crystallite is higher than that of the internal energy up to a certain radial distance of the dopant, after which the rate of change in the entropy is lower. This gives rise to a free energy barrier which leads to internalization of the dopant when it is within a certain radial distance and its expulsion when it is outside this region.
Our experimental system consists of a collection of colloidal particles and a dopant in a highly configurable confining potential, formed using a defocussed optical tweezer. By varying the power and level of defocussing, colloidal assemblies of different sizes and phases (liquid-like or crystalline) could be formed. Most importantly, we have studied the dynamics of foreign dopants, in the form of particles of different shapes and sizes, injected into the crystallites. The striking result obtained here was the ability of finite-sized colloidal clusters to expel or internalize a foreign dopant depending on its initial position. This surprising phenomenon could be reproduced in our simulations which also provided an explanation of this behaviour. We found that the fate of a dopant is governed to a large extent by the entropy of the system, which becomes increasingly important as the size of the crystallite is reduced. The internal energy of the system was found to decrease as the dopant gets expelled, which by itself would imply expulsion of the dopant for all initial positions. However, the entropy of the system also decreases as the dopant is expelled, because the system gets more ordered in this process. This effect favours internalization of the dopant. Careful observations show that the rate of change of the entropy with increasing distance of the dopant from the centre of the crystallite is higher than that of the internal energy up to a certain radial distance of the dopant, after which the rate of change in the entropy is lower. This gives rise to a free energy barrier which leads to internalization of the dopant when it is within a certain radial distance and its expulsion when it is outside this region.
The studies presented here can be extended to host colloids of higher degree of complexity, e.g. non-spherical shapes, self-propelled particles etc, and the insight gained from such studies would be useful in designing and assembling new types of soft nanomaterials.
Other Featured research




 The physics of strongly confined systems of interacting particles has been a subject of active research for more than a century. These are systems consisting of a small number of particles confined in a small region by an external potential or small clusters formed due to inherent inter-particle interactions. Such systems are ubiquitous in nature – examples include petroleum in porous rocks, cytoplasm in biological cells and electrons in a quantum dot. Physical properties of such systems are substantially different from those of macroscopic (bulk) systems because the fraction of particles on the surface of a small cluster is larger than that for a bulk system and this fraction increases as the size of the cluster is reduced. Also, small local changes inside a cluster, such as the presence of a single impurity or the formation of a single defect, can affect the properties of the whole cluster if it is small. For these reasons, physical processes such as crystallization, glass formation and defect dynamics in strongly confined systems can exhibit characteristics that are fundamentally different from those found in their bulk counterparts. The development of an understanding of these effects of strong confinement is of fundamental importance in the design and assembly of nanomaterials.
The physics of strongly confined systems of interacting particles has been a subject of active research for more than a century. These are systems consisting of a small number of particles confined in a small region by an external potential or small clusters formed due to inherent inter-particle interactions. Such systems are ubiquitous in nature – examples include petroleum in porous rocks, cytoplasm in biological cells and electrons in a quantum dot. Physical properties of such systems are substantially different from those of macroscopic (bulk) systems because the fraction of particles on the surface of a small cluster is larger than that for a bulk system and this fraction increases as the size of the cluster is reduced. Also, small local changes inside a cluster, such as the presence of a single impurity or the formation of a single defect, can affect the properties of the whole cluster if it is small. For these reasons, physical processes such as crystallization, glass formation and defect dynamics in strongly confined systems can exhibit characteristics that are fundamentally different from those found in their bulk counterparts. The development of an understanding of these effects of strong confinement is of fundamental importance in the design and assembly of nanomaterials. Our experimental system consists of a collection of colloidal particles and a dopant in a highly configurable confining potential, formed using a defocussed optical tweezer. By varying the power and level of defocussing, colloidal assemblies of different sizes and phases (liquid-like or crystalline) could be formed. Most importantly, we have studied the dynamics of foreign dopants, in the form of particles of different shapes and sizes, injected into the crystallites. The striking result obtained here was the ability of finite-sized colloidal clusters to expel or internalize a foreign dopant depending on its initial position. This surprising phenomenon could be reproduced in our simulations which also provided an explanation of this behaviour. We found that the fate of a dopant is governed to a large extent by the entropy of the system, which becomes increasingly important as the size of the crystallite is reduced. The internal energy of the system was found to decrease as the dopant gets expelled, which by itself would imply expulsion of the dopant for all initial positions. However, the entropy of the system also decreases as the dopant is expelled, because the system gets more ordered in this process. This effect favours internalization of the dopant. Careful observations show that the rate of change of the entropy with increasing distance of the dopant from the centre of the crystallite is higher than that of the internal energy up to a certain radial distance of the dopant, after which the rate of change in the entropy is lower. This gives rise to a free energy barrier which leads to internalization of the dopant when it is within a certain radial distance and its expulsion when it is outside this region.
Our experimental system consists of a collection of colloidal particles and a dopant in a highly configurable confining potential, formed using a defocussed optical tweezer. By varying the power and level of defocussing, colloidal assemblies of different sizes and phases (liquid-like or crystalline) could be formed. Most importantly, we have studied the dynamics of foreign dopants, in the form of particles of different shapes and sizes, injected into the crystallites. The striking result obtained here was the ability of finite-sized colloidal clusters to expel or internalize a foreign dopant depending on its initial position. This surprising phenomenon could be reproduced in our simulations which also provided an explanation of this behaviour. We found that the fate of a dopant is governed to a large extent by the entropy of the system, which becomes increasingly important as the size of the crystallite is reduced. The internal energy of the system was found to decrease as the dopant gets expelled, which by itself would imply expulsion of the dopant for all initial positions. However, the entropy of the system also decreases as the dopant is expelled, because the system gets more ordered in this process. This effect favours internalization of the dopant. Careful observations show that the rate of change of the entropy with increasing distance of the dopant from the centre of the crystallite is higher than that of the internal energy up to a certain radial distance of the dopant, after which the rate of change in the entropy is lower. This gives rise to a free energy barrier which leads to internalization of the dopant when it is within a certain radial distance and its expulsion when it is outside this region.