Sustainable and Selective Synthesis of Drug-like N-Aryl Phthalimides/Maleimides
– Parth Kumar
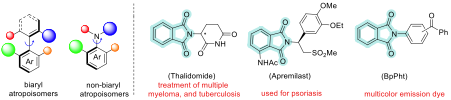
Atropisomers are a type of isomer that arises due to restricted rotation around a bond, resulting in non-superimposable mirror images. Researchers from the Department of Organic Chemistry led by AT Biju have devised a sustainable method to selectively synthesise individual atropisomers of compounds called N-aryl phthalimides and maleimides. Structurally similar molecules are used in drugs to treat many diseases. For instance, thalidomide and apremilast, both featuring the phthalimide skeleton, are well-known drugs used for the treatment of multiple myeloma, tuberculosis, and psoriatic arthritis. Different isomers of the same molecules in drugs can have different pharmacological effects on the body, which is why atropisomeric purity is important. Moreover, N-aryl phthalimide derivatives are widely applicable as catalysts, dyes, and for the synthesis of polymers.
Traditional methods to synthesise N-aryl phthalimides require harsh conditions and expensive metal catalysts. The new synthetic method requires milder conditions and low amounts of catalyst and is hence more sustainable. Instead of using traditional metal catalysts, the researchers utilised organic catalysts called N-heterocyclic carbenes (NHCs), which form a reactive intermediate, finally resulting in the sustainable and atroposelective method to access a library of such molecules. For the first time, the team used an acid activation strategy to make nitrogen-carbon bonds in the presence of NHCs under mild conditions, to drive the reaction to completion. Using this strategy, researchers synthesised a wide variety of structurally intriguing N-aryl phthalimides and maleimides with high atropisomeric purity under mild conditions.
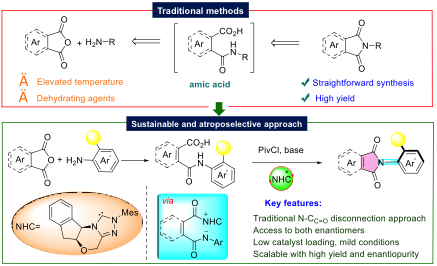
Interestingly, both atropisomers of the phthalimides can be accessed starting from the same substrates (phthalic anhydride and aniline) using the same NHC, which is not common in organocatalytic reactions.
With diverse applications in making drugs, dyes and catalysts, this development is likely another step forward in green chemistry.

From left to right: AT Biju, Soumen Barik, Sowmya Shree R
REFERENCE:
Barik S, Ranganathappa SS, Biju AT, N-heterocyclic carbene-catalyzed atroposelective synthesis of N-Aryl phthalimides and maleimides via activation of carboxylic acids, Nature Communications (2024).
https://doi.org/10.1038/s41467-024-49799-5
LAB WEBSITE:
https://atbiju.in/






