Enhanced water evaporation from Ȧ-scale nanoporous graphene
With growing global water stress, there is a pressing need to develop potable water technologies that have a lower carbon footprint. In a new study, researchers from the Department of Chemical Engineering (CE), IISc and collaborators have developed a novel, low thermal input, evaporation-based process using single layered graphene sheets synthesised with tunable-sized nanopores.
Enhancing the kinetics of liquid–vapor transition from nanoscale confinements is an attractive strategy for developing evaporation and separation applications. The ultimate confinement limit for evaporation is an atom-thick interface hosting angstrom-scale nanopores.
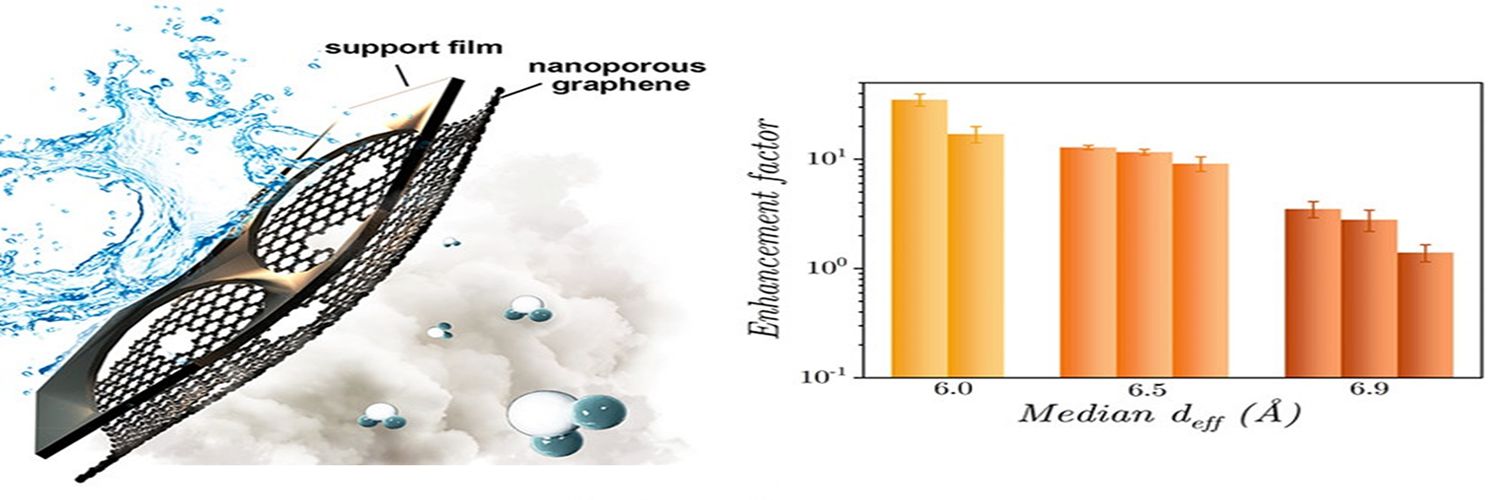
In this regard, for the first time, the researchers have reported a 35-fold enhancement in water evaporation rates from Ȧ-scale graphene nanopores relative to a bare liquid-vapor interface. The team included K Ganapathy Ayappa, Professor in CE, and his PhD student Anshaj Ronghe, in collaboration with Kumar Varoon Agrawal, and Wan Chi Lee with their group at the Institute of Chemical Sciences and Engineering, École Polytechnique Fédérale de Lausanne (EPFL), Switzerland.
Using a vapor deposition process, the group at EPFL synthesised nanoporous graphene and studied the water evaporation rates as a function of the nanopore dimensions. This is in sharp contrast to earlier studies where evaporation had been studied through channels, presenting a significant advance in the development of nanopore-based technologies.
The group at IISc carried out extensive molecular dynamics simulations using the supercomputing resources at the Supercomputer Education and Research Center to provide molecular insights to explain this enhancement.
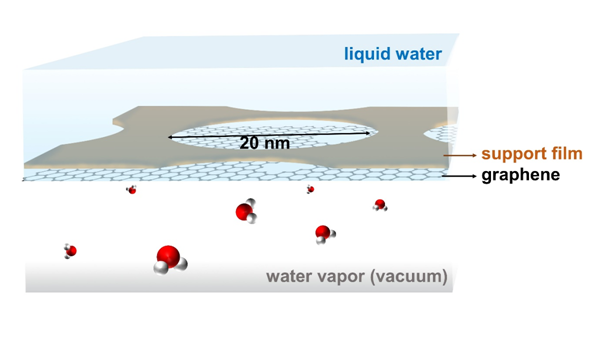
Setup to study enhanced evaporation from nanoporous graphene
Molecular dynamics simulations allow one to track the velocities and positions of water molecules as they exit the nanopore from the liquid into the vapor phase. Edge functionalised graphene nanopores were found to render reduced and short-lived water-water hydrogen bonds in the vicinity of the nanopore, resulting in a higher escaping tendency of water molecules into the vapor phase. These simulations revealed that evaporation varies inversely with the nanopore size, with hydrophilic edge functionalised nanopores yielding higher evaporation enhancements. Reduced free energy barriers and loweredsurface tension at the liquid-vapor interface correlated with the higher evaporation tendency of water molecules observed in the experiments withnanoporous graphene.Challenges with nanopore-based technologies lie in developing effective strategies for larger scale and commercially viable processes, and this is currently being explored.
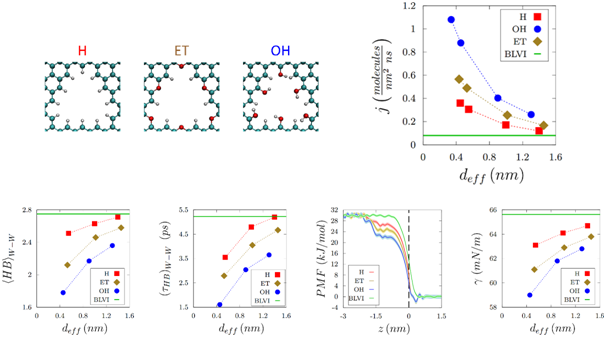
Top panel – Left to right: Nanoporous graphene terminated with various functional groups (H atoms, Ether functional groups, and Hydroxyl functional groups). Evaporation flux from functionalized nanoporous graphene with different sized nanopores. Bottom panel – Left to right: Number of water-water hydrogen bonds. Water-water hydrogen bond lifetimes. Potential of mean force profiles for water evaporation. Surface tension at the liquid-vapor interface

Left to right: K Ganapathy Ayappa and Anshaj Ronghe, Department of Chemical Engineering,IISc

Left to right: Kumar Varoon Agrawal and Wan-Chi Lee (EPFL, Switzerland)
REFERENCE:
Lee WC, Ronghe A, Villalobos LF, Huang S, Dakhchoune M, Mensi M, Hsu
KJ, Ayappa KG and Agrawal KV, Enhanced Water Evaporation from Å-Scale
Graphene Nanopores, ACS Nano (2022).
https://pubs.acs.org/doi/full/10.1021/acsnano.2c07193
LAB WEBPAGE:
https://kgalabiisc.wixsite.com/kgalab








