Symmetry Breaking Approach to a Unique Natural Product
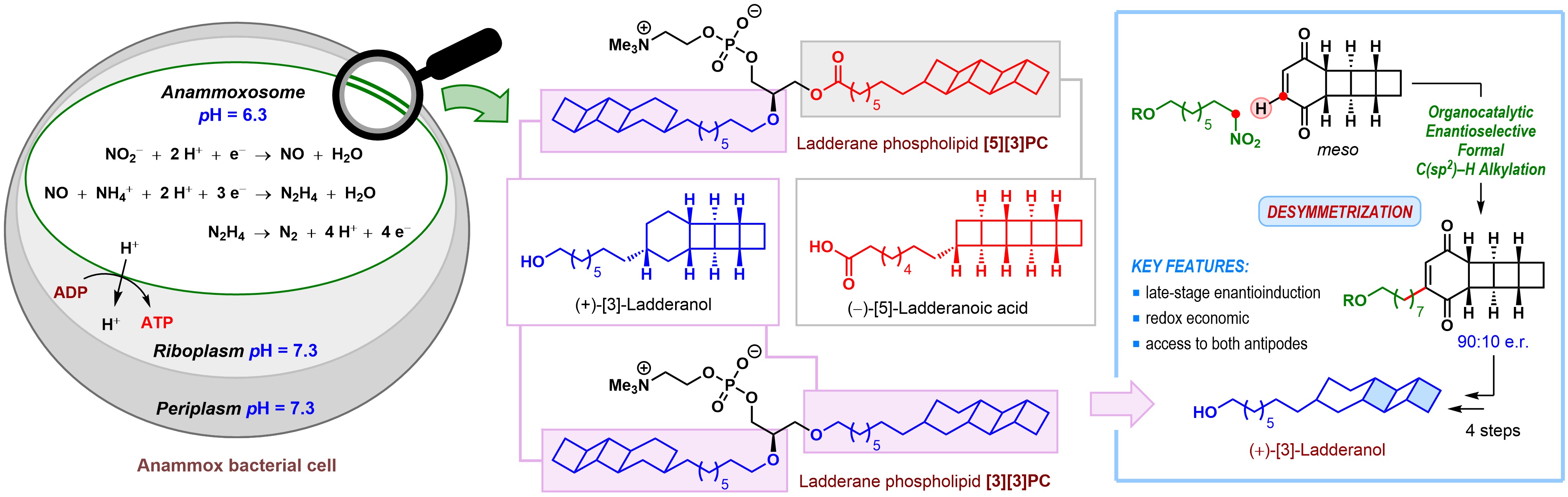
Architecturally unique compounds produced by nature have remained a source of inspiration for organic chemists. Ladderane phospholipids (such as [5][3]PC and [3][3]PC where PC denotes phosphatidylcholine), isolated in the year 2002 from anaerobic ammonium oxidizing (anammox) bacteria, revealed such never-seen-before structural features in nature. These molecules were named so due to the presence of highly unusual ladder-like arrangements of concatenated cyclobutane rings. These ladderane phospholipids are composed of either (+)-[3]-ladderanol or a mixture of (+)-[3]-ladderanol and (–)-[5]-ladderanoic acid.
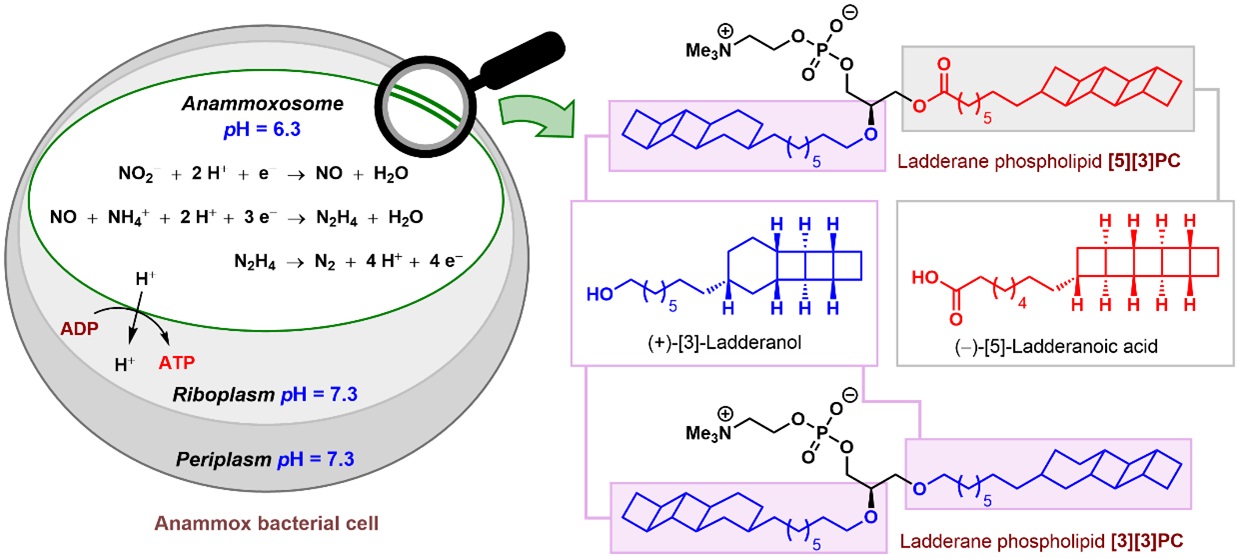
These phospholipids appear to play an important role in the function of anammox bacteria. In a large intracellular compartment of these bacteria, known as the anammoxosome, ammonium and nitrite are coupled to produce dinitrogen in an overall exergonic reaction called the anammox process. The presence of ladderane phospholipids in the membrane of anammoxosome imparts unusual density, which helps in maintaining a pH gradient across the membrane that drives ATP synthesis.
Due to the slow growth rate of the producing organism and the inherent difficulty in purifying such complex lipid mixtures, laboratory synthesis remains the primary source of ladderane phospholipids for studying the relationship between their structure and biological function.
A research team from the Department of Organic Chemistry led by Santanu Mukherjee has now developed a symmetry-breaking approach to [3]-ladderanol. The team has applied an organocatalytic enantioselective formal C(sp2)‒H alkylation reaction, developed in their laboratory in 2015, to the symmetric tetracyclic carbon skeleton of [3]-ladderanol. This key C‒C bond construction step is accomplished in a one-pot two-step operation, under a sequential combination of a chiral tertiary aminosquaramide and an achiral tertiary aminourea as catalysts.
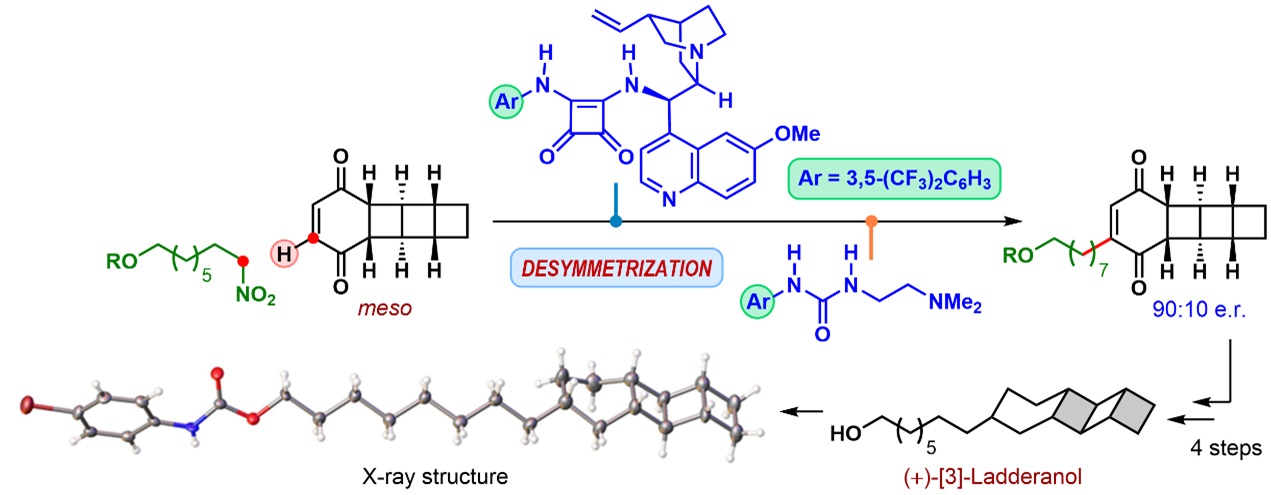
This is the first time that an enantioselective desymmetrization strategy is applied to the synthesis of [3]-ladderanol. This synthetic strategy benefits from the late-stage introduction of chirality as it offers flexibility to the already concise route and allows for the facile access to both enantiomers of [3]-ladderanol as well as an analogue.
Reference:
S. Ray, S. Mondal and S. Mukherjee, Enantioselective Total Synthesis of [3]-Ladderanol through Late-Stage Organocatalytic Desymmetrization Angewandte Chemie International Edition, 2022, 61, e202201584. https://doi.org/10.1002/anie.202201584
M. S. Manna and S. ournal of the American Chemical Society 2015, 137, 130-133. https://doi.org/10.1021/ja5117556ournal of the American Chemical Society 2015, 137, 130-133. https://doi.org/10.1021/ja5117556
Lab website:
https://orgchem.iisc.ac.in/santanu_mukherjee/SM.htm
The Team:

From left to right: Sayan Ray, Santanu Mukherjee and Subhajit Mondal







