Tiny lithium packs a mighty punch
Ocean acidification is the twin brother of human induced global warming. At present, approximately 40% of the total CO2 emitted from burning of fossil fuel is absorbed by seawater, and the rest remains in the atmosphere. This phenomenon dampens the CO2 driven greenhouse warming and results in acidification of seawater. This is because; CO2 is a reactive gas that readily dissolves in seawater to form carbonic acid (H2CO3). Additionally, the coupled nature of atmospheric CO2 concentration and seawater acidity implies that over the history of the planet these two parameters have co-varied.
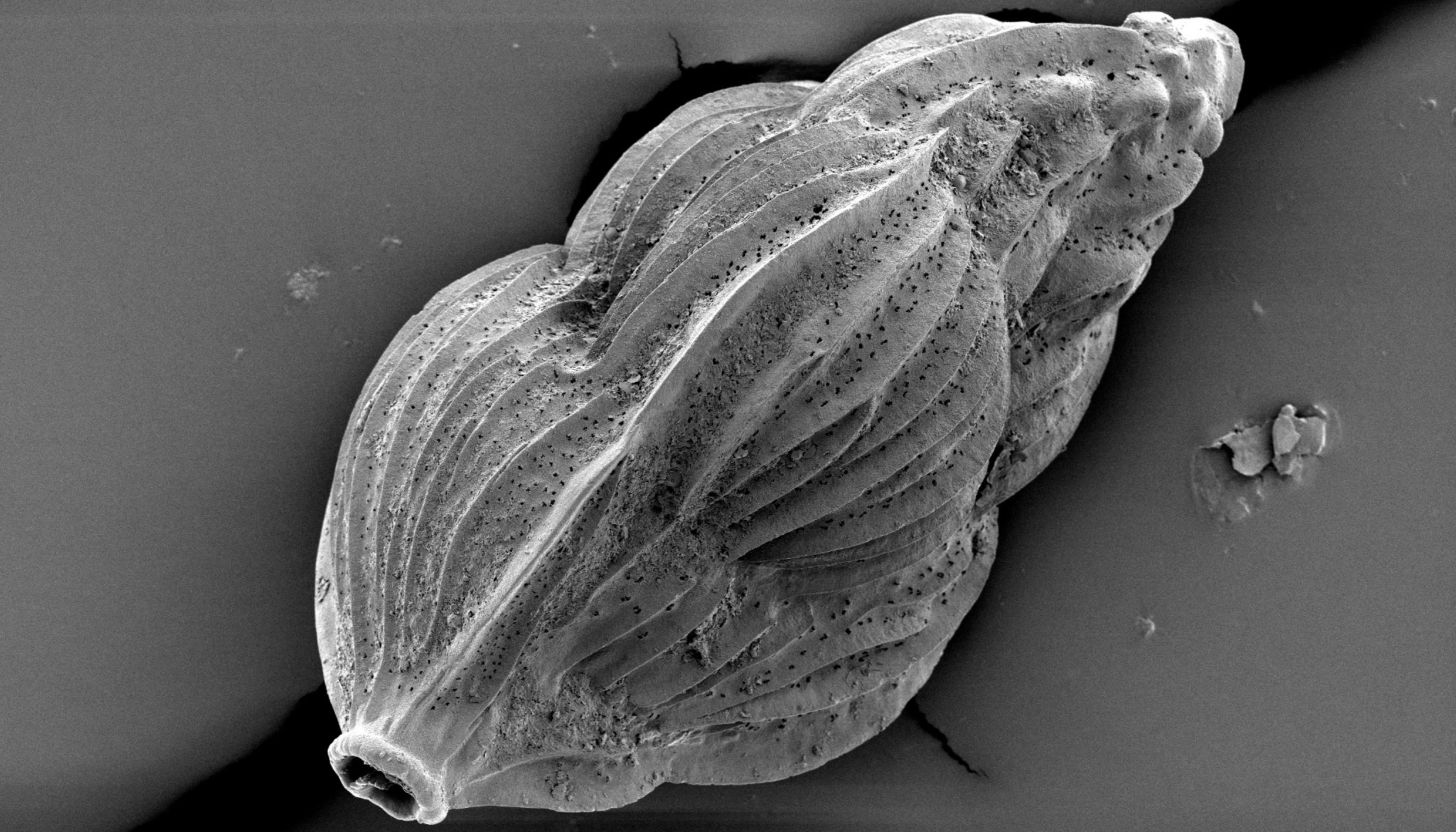
Secondary electron microscope (SEM) image of Angulogerina angulosa, an in-faunal benthic foraminifera. These deep dwelling, calcium carbonate secreting organisms live inside (in-faunal) the sediment at the bottom of the ocean (benthic), under very fast moving currents, and are ~0.3 millimeter long.

Principal Investigator: Sambuddha Misra
The deep-ocean, the cold and dense waters of the abyss, contains approximately nine times more dissolved CO2 than the atmosphere. The exchange of CO2 between the deep-ocean and atmosphere, caused by density driven ocean circulation, is the key modulator of atmospheric CO2 levels and thus, global temperature. To understand the role deep-ocean ventilation in natural climate change, and to assess the severity and timing of ocean acidification caused by anthropogenic CO2 emission a temporal record of abyssal seawater pH is critical.
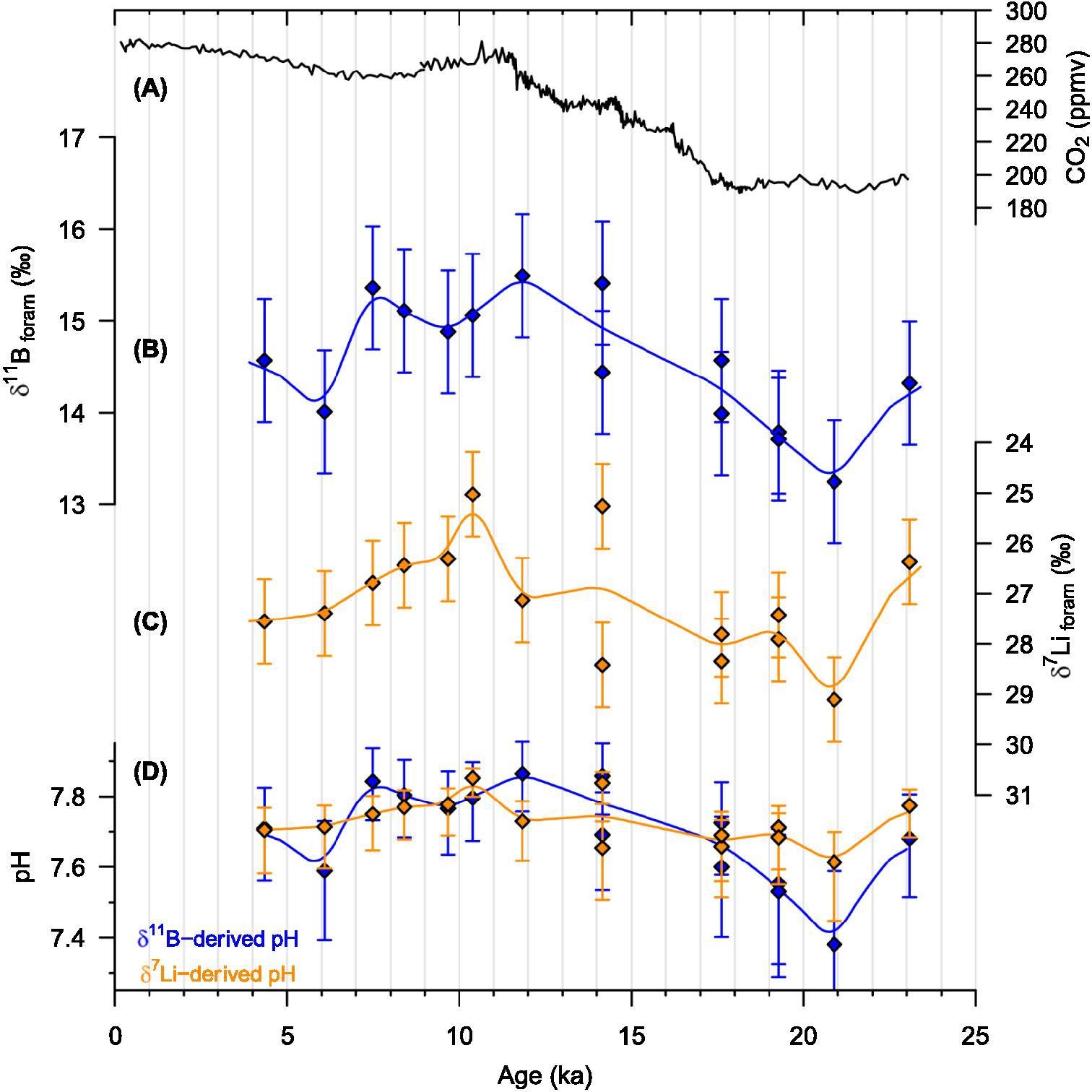
Records of last Deglacial, from the last glacial maxima 20,000 years ago to modern day, lithium (δ7Li) and boron (δ11B) from a sediment core collected from the deep South Pacific. The two isotopic records were generated using specimens of Cibicidoides mundulus (100–300 μg) that were picked from a gravity core collected from 3581 m water depth of the deep South Pacific (55°09.74′S; 114°47.31′W). The core is located on the eastern flank of East Pacific Rise about 300 km north of the Sub-Antarctic Front. Cibicidoides mundulus were chemically cleaned to get to the pristine calcite, following that sub-nano gram levels of lithium and boron were purified from each samples, and then precise isotope ratio determination of the two elements were done on a multi collector inductively coupled plasma mass spectrometer (MC-ICP-MS) at University of Cambridge. The boron isotopic composition (δ11B) of foraminifera is an established proxy for seawater pH. The panel (A) shows the atmospheric CO2 concentration derived from Antarctic ice core samples; the last Deglacial Cibicidoides mundulus δ11B and δ7Li values are shown in panel (B) and (C) respectively (Note the reversed y-axis); panel (D) compares the δ11B- and δ7Li-derived pH, blue and orange curves respectively. Analytical uncertainty (2σ) is based on homogenised replicate measurements of the sample (where available) or on replicate measurements of an external coral standard.
Microscopic carbonate-secreting organisms living in seawater, like coccoliths and foraminifera, incorporates trace quantities of dissolved elements from seawater into their calcium carbonate shells during their lifetime. We discovered that the isotopic composition of lithium, ratio of 7Li to 6Li (both stable in nature), trapped within the calcium carbonate lattice is strongly correlated with pH of the organism’s growth environment. We plan to apply this new proxy for seawater pH to accurately determine the timing, rate, and extend of natural and anthropogenic alteration of the CO2 budget of the ocean-atmosphere system!
Click here for an Additional Figure about ‘The pH and dissolved inorganic carbon (DIC) dependence of lithium isotopic composition (δ7LiSHELL) of cultured Amphistegina lessonii. Culture experiments were performed by decoupling the pH and the carbonate chemistry of the matrix at the Alfred Wegener Institute in Germany. Please see legend for pH and [CO3=] (µmol/kg) conditions of each treatment. The 2σ error of pH and [DIC] based on replicate measurements of the culture matrix was 0.1 pH units and 20 μmol/kg, respectively. The grey bar represents the 2σ external uncertainty of δ7LiMATRIX based on triplicate measurements’.
References:
- https://www.sciencedirect.com/science/article/pii/S0016703718301200
Roberts, J., Kaczmarek, K., Langer, G., Skinner, L.C., Bijma, J., Bradbury, H., Turchyn, A.V., Lamy, F. and Misra, S., 2018. Lithium isotopic composition of benthic foraminifera: A new proxy for paleo-pH reconstruction. Geochimica Et Cosmochimica Acta.
- https://onlinelibrary.wiley.com/doi/full/10.1002/rcm.8020
Bohlin, M.S., Misra, S., Lloyd, N., Elderfield, H. and Bickle, M.J., 2018. High‐precision determination of lithium and magnesium isotopes utilising single column separation and multi‐collector inductively coupled plasma mass spectrometry. Rapid Communications in Mass Spectrometry, 32(2), pp.93-104.
- https://onlinelibrary.wiley.com/doi/full/10.1002/rcm.8009
Lloyd, N.S., Sadekov, A.Y. and Misra, S., 2018. Application of 1013 ohm Faraday cup current amplifiers for boron isotopic analyses by solution mode and laser ablation multicollector inductively coupled plasma mass spectrometry. Rapid Communications in Mass Spectrometry, 32(1), pp.9-18.
Website: https://scholar.google.co.uk/citations?user=DDQpOdEAAAAJ&hl=en






