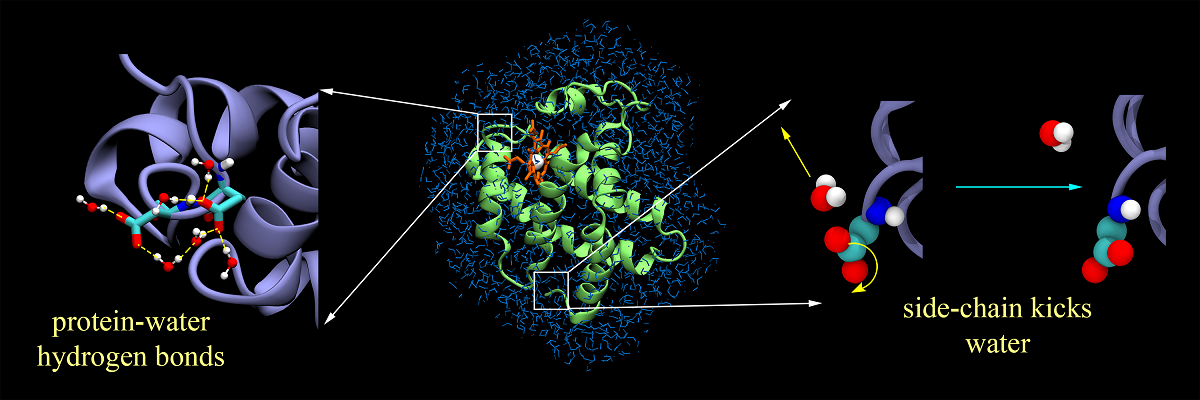
Water slaves protein dynamics by action at a distance
The coupling between protein and water remains a topic of extensive debate, with the main emphasis being the role of water in protein’s function. This work offers a completely new perspective on this problem in terms of coupled energy fluctuations. Here we present two novel results that help in better understanding the issue. Firstly, we discover an important hitherto unknown correlation between protein and water dynamics. We observe that energy fluctuations of protein and water are anti-correlated. The extra-ordinarily low dielectric constant of protein allows efficient transmission of polar interaction between dipolar/polar groups in protein and the surrounding water molecules. This results in a flow of energy from water to protein that can be channelized for function. The second remarkable result is the bimodal energy spectrum of protein, showing two distinct 1/f noise characteristics. Clear signatures of water induced dynamic perturbation fortify the notion of “solvent slaving of protein dynamics”. Hence, our results provide a unique viewpoint toward this well-studied yet still poorly understood problem of protein-water coupled interactions.
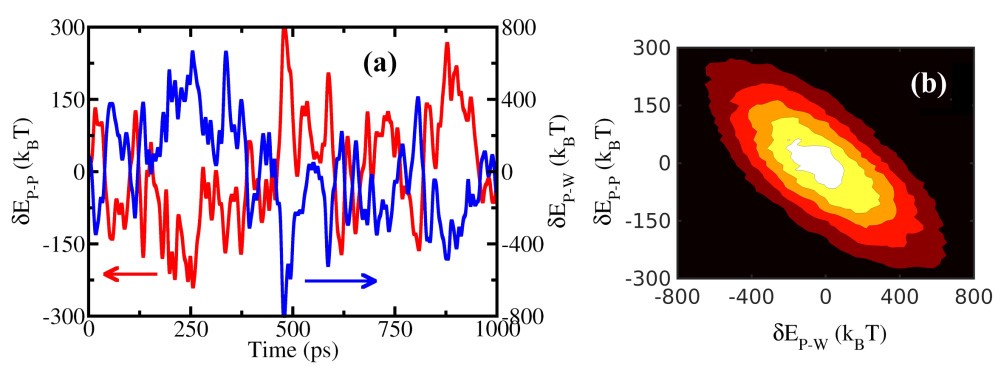
(a) Protein (myoglobin) self-energy fluctuations (dEP-P) and protein-water interaction energy fluctuations (dEP-W); (b) contour representation of the bivariate distribution of these two energy contributions. The energy trajectories and contour diagram denote strong anti-correlation. Similar results are obtained for other proteins (lysozyme, plastocyanin, insulin, HP-36).
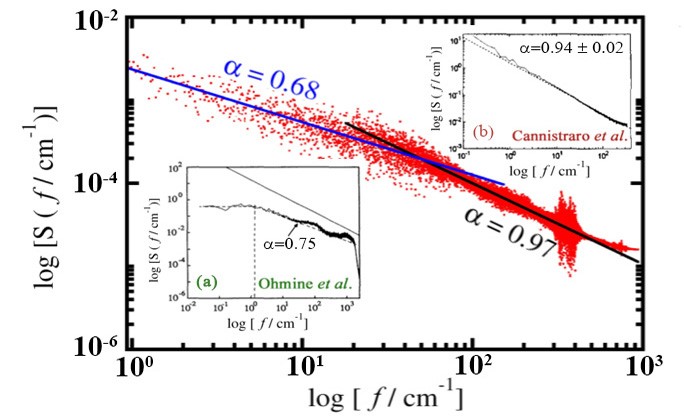
1/f noise behaviour of the spectral density of total energy fluctuations in plastocyanin. In presence of interaction with water, the power spectrum shows two slopes, one corresponding to the protein, and the other due to water. The spectra for water and plastocyanin are shown in insets (a) and (b) respectively.

Bagchi group
References
Phys. Rev. Lett. January 2019 https://journals.aps.org/prl/accepted/f3076Y14F471c16826f81f918fc2d5594dbf1feaa
Website : Biman Bagchi







