 An interdisciplinary team of IISc researchers has designed a novel chemical compound that can be tailored to release aspirin over a desired duration inside the body. It has several advantages over existing mechanisms: it is harmless to living cells, can carry a large amount of drug, and can easily be injected.
An interdisciplinary team of IISc researchers has designed a novel chemical compound that can be tailored to release aspirin over a desired duration inside the body. It has several advantages over existing mechanisms: it is harmless to living cells, can carry a large amount of drug, and can easily be injected.
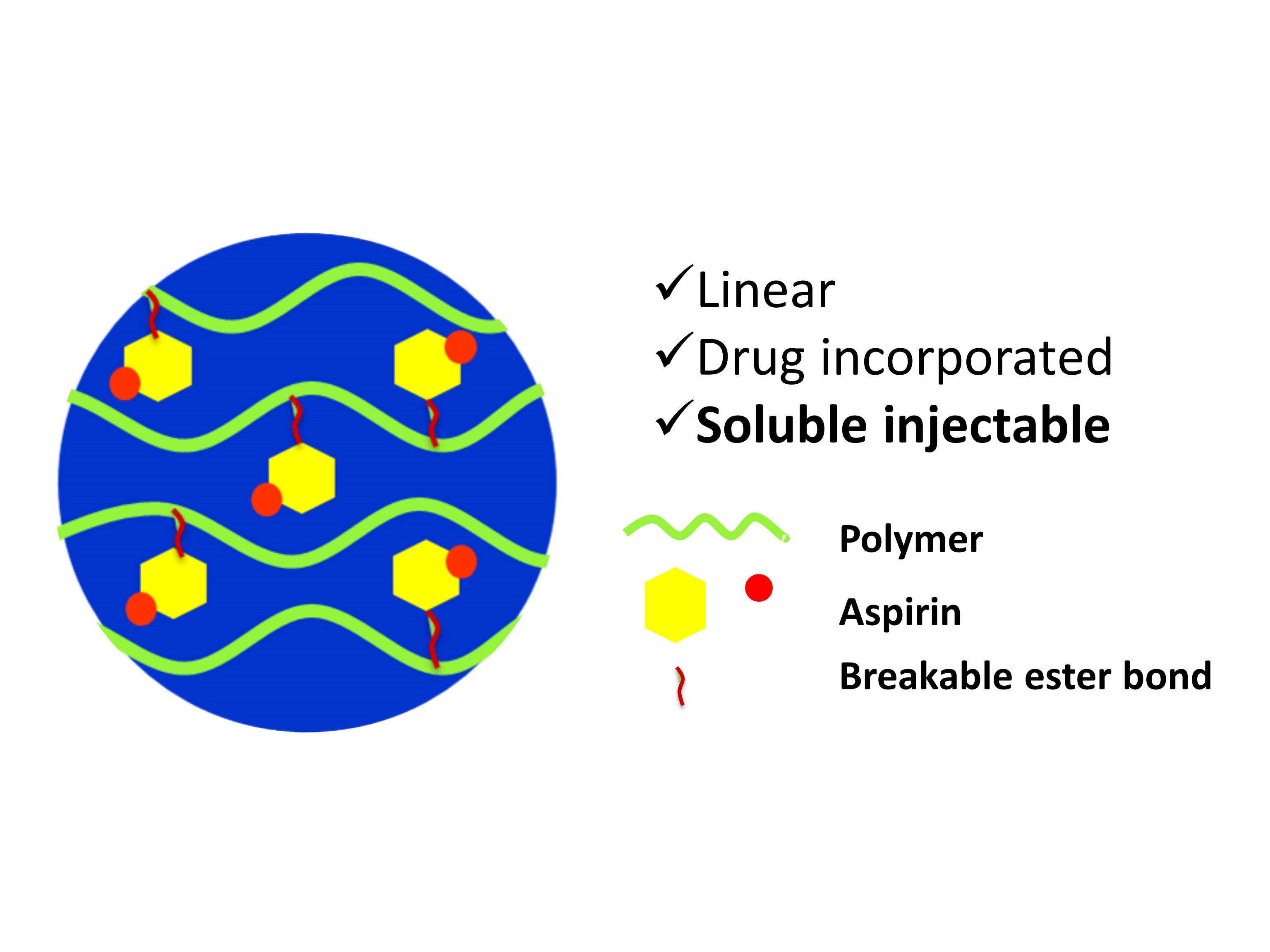
Commonly used pills or patches have aspirin trapped inside a capsule or a mesh. The new compound, however, has aspirin built directly into the backbone of a long polymer chain with a sugar molecule called xylitol. Xylitol is naturally found in fruits and vegetables, and is used as a sweetener. The bond between xylitol and the drug will slowly be broken down by the body’s enzymes, allowing the drug to gradually diffuse into the bloodstream.
“Unlike your pill which will release all the drug at the same time, this would allow the molecule to circulate longer and have a more prolonged effect,” says Kaushik Chatterjee, Associate Professor, Department of Materials Engineering, one of the authors of the paper published in the International Journal of Pharmaceutics.
Aspirin is best known for reducing fever and pain. This household drug can also lower the risk of heart attack or stroke by preventing clots from forming in blood vessels. Previous research has shown that a gradual release of aspirin instead of a rapid initial burst can stave off unwanted side effects. A single compound that circulates in the body and releases a certain amount of aspirin periodically may also provide an alternative to taking multiple pills at different times.
In recent years, scientists have turned to polymers — large molecules with repeating units — to develop slow-release systems with the drug built in. Ideally, such a compound should also be harmless to living cells and be able to carry a large amount of drug.
In this study, the researchers picked a sugar alcohol called xylitol as its base because it meets all the above requirements. For one, it forms degradable polymers that can break down slowly inside the body, giving the drug a longer time to diffuse. It also has more free sites for the drug to bind than other alcohols.
“Another major advantage is that it is entirely biodegradable,” says first author Queeny Dasgupta, PhD student at the Centre for Biosystems Science and Engineering. “All the precursors used to make the polymer are endogenous (native) to the body so there is no accumulation.” Xylitol is also relatively unreactive and unlikely to interfere with aspirin’s action.
However, previously developed xylitol-based polymers have a drawback. During synthesis, they form complex mesh-like structures (crosslinked polymers) that are difficult to dissolve and inject, and have to be surgically implanted inside the body.
In the current study, the researchers modified the reaction conditions in such a way that xylitol was forced to form a long, straight chain that can easily be dissolved into a gel and injected. “The linear chain is important because only then can you inject it. Once it forms a network, it becomes a solid lump that is implantable but not injectable,” says Chatterjee. The linear polymerization was achieved using a novel technique.
The researchers found that their compound was able to release about 8% of the drug in the first week, 13% in the second and 20% by four weeks. When tested with blood cells in the lab, the released drug was able to reduce inflammation — a key reaction in reducing pain — as expected.
By tweaking the type of chemical bonds and number of free sites that the compound has, the timing and dose of drug delivery can be adjusted, the authors say. “For example, if you want low doses of aspirin over sustained periods, say weeks, then you may want to have more such bonds that would not degrade so easily,” says Chatterjee.
The technique can also be used to develop similar delivery vehicles for other drugs with sustained and tunable release, the researchers say.
Reference: Controlled release from aspirin based linear biodegradable poly(anhydride esters) for anti-inflammatory activity, published in International Journal of Pharmaceutics, August 2017.
http://www.sciencedirect.com/science/article/pii/S0378517317305732


 An interdisciplinary team of IISc researchers has designed a novel chemical compound that can be tailored to release aspirin over a desired duration inside the body. It has several advantages over existing mechanisms: it is harmless to living cells, can carry a large amount of drug, and can easily be injected.
An interdisciplinary team of IISc researchers has designed a novel chemical compound that can be tailored to release aspirin over a desired duration inside the body. It has several advantages over existing mechanisms: it is harmless to living cells, can carry a large amount of drug, and can easily be injected.