11 November 2024
– Rohini Subrahmanyam
Most of the disease-causing bacteria secrete matrix-like layers around themselves called biofilms. These biofilms act as thick barriers limiting the entry of drugs and helping the bacteria become resistant to antibiotics.
Now, a team of scientists at the Indian Institute of Science (IISc) has devised a way to break down this biofilm barrier, using an enzyme from the cow’s digestive tract. The findings were published in npj Biofilms and Microbes.
Klebsiella pneumoniae is an opportunistic bacterium that infects patients in hospitals, causing conditions like pneumonia, urinary tract infections, and meningitis. In people with diabetes, it infects wounds which end up healing slowly, leading to complications that may sometimes result in amputation of a limb.
K. pneumoniae secretes a tough matrix-like biofilm made up mostly of sugars, fats, proteins, and DNA. Strings of sugar molecules in this matrix come together to form polysaccharides, which play a key role in making the biofilm stronger.
The IISc team decided to develop a biocompatible strategy to break down these polysaccharides and disrupt the protective biofilm. They realised that polysaccharide-degrading enzymes could serve the purpose and that a cow’s gut was the ideal place to look for them. The bovine gut harbours microbial enzymes that digest different kinds of complex polysaccharides, such as cellulose and hemicellulose, which the animal takes in from its plant food.
Cellulosic polysaccharides are very similar to the ones in the bacterial biofilms. The team focused on a set of enzymes called glycoside hydrolases (GH), looking closely at their protein structures to identify the ones that could potentially break down polysaccharides efficiently. They zoomed in on one they called GH-B2, found in the rumen, the largest stomach compartment, and artificially synthesised it in the lab.
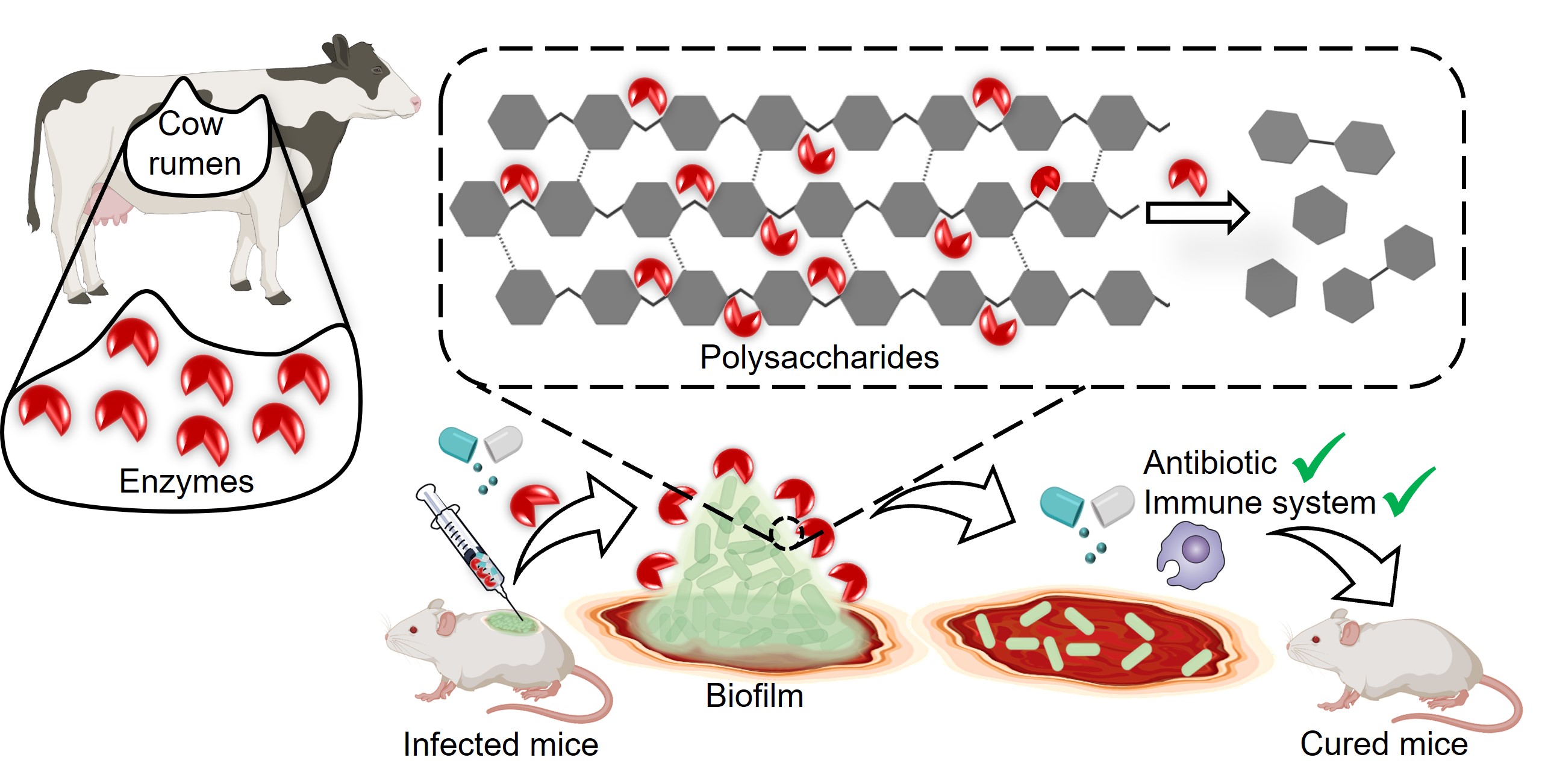
Enzymatic dispersion of K. pneumoniae biofilm ─ enhancing antibiotic and immune system efficacy, leading to curing infection (Image: Reshma Ramakrishnan)
When they tested the lab-made GH-B2 on four different strains of K. pneumoniae isolated from different hospital patients, they found that it successfully broke down biofilms in all four.
“Such broad activity of the enzyme was surprising, as the strains were different in terms of their serotypes,” says Debasis Das, Assistant Professor in the Department of Inorganic and Physical Chemistry (IPC) and corresponding author of the study. Different strains of the same bacteria respond differently to the host’s immune cells, leading to variants called serotypes.
The researchers found that treating a mature biofilm with the enzyme – resulting in collapse of the biofilm matrix – made the bacteria 15 times more susceptible to a broad-spectrum antibiotic called meropenem. When they added the enzyme at the start of biofilm formation, the enzyme prevented the biofilm from developing altogether, and the bacteria became 250 times more susceptible to the antibiotic. The biofilm breakdown also made K. pneumoniae more vulnerable to attack by immune cells from mice and humans.
The team also tested their enzyme in lab mice that had wounds infected by K. pneumoniae. Treating the wound with both the enzyme and meropenem caused the biofilms to clear up completely.
“The chances of [the bacteria] gaining resistance are very less because our enzyme is not harming the bacteria directly; it is just disrupting the matrix,” says Reshma Ramakrishnan, PhD student in Das’s lab and first author of the study. “[This] will be a combinatorial therapy – we can use the enzyme plus the antibiotic for treating K. pneumoniae biofilm-associated infections.”
The researchers are now planning on incorporating the enzyme in something wearable, like a wound dressing. “[For] local infections, specifically skin infections in diabetic individuals, this can be very promising,” says Dipshikha Chakravortty, Professor at the Department of Microbiology and Cell Biology (MCB) and another corresponding author of the study.
Das suggests that the enzyme can also be used as a coating material on medical devices to prevent biofilm formation.
“We need to make newer strategies to break the lifestyle of bacteria,” says Chakravortty. “It is important for us to continuously look out for these potent enzymes to combat infectious diseases which are currently a threat.”

The research team from left to right: Debasis Das, Reshma Ramakrishnan, Kirti Parmar, Dipshikha Chakravortty, Abhilash V Nair, Raju S Rajmani (Photo: Jayaprakash K)
REFERENCE:
Ramakrishnan R, Nair A, Parmar K, Rajmani RS, Chakravortty D, Das D, Combating biofilm-associated Klebsiella pneumoniae infections using a bovine microbial enzyme, npj Biofilms and Microbiomes (2024).
https://www.nature.com/articles/s41522-024-00593-7
CONTACT:
Dipshikha Chakravortty
Professor
Department of Microbiology and Cell Biology (MCB)
Indian Institute of Science (IISc)
Email: dipa@iisc.ac.in
Phone: +91 80 2293 2842
Website: https://mcb.iisc.ac.in/dclab/
Debasis Das
Assistant Professor
Department of Inorganic and Physical Chemistry (IPC)
Indian Institute of Science (IISc)
Email: debasisdas@iisc.ac.in
Phone: +91 80 2293 3002
Website: https://sites.google.com/view/ddlaboratory/home
NOTE TO JOURNALISTS:
a) If any of the text in this release is reproduced verbatim, please credit the IISc press release.
b) For any queries about IISc press releases, please write to news@iisc.ac.in or pro@iisc.ac.in.



