21 May 2025
– Parth Kumar
From anti-counterfeiting inks to bioimaging agents, phosphorescent materials that glow after light exposure are critical for security and sensing applications. However, most current systems rely on inorganic phosphors or heavy metal complexes, which are expensive and biologically incompatible, and are not environment-friendly. Among organic materials, achieving long-lasting phosphorescence at room temperature remains exceptionally rare. Moreover, integrating circularly polarised luminescence (CPL) – useful for advanced display and encryption technologies – with phosphorescence in small organic molecules has remained largely elusive.
A team of researchers at the Department of Inorganic and Physical Chemistry (IPC), Indian Institute of Science (IISc) has addressed these challenges by designing a new chiral aminoborane molecule that exhibits persistent room-temperature phosphorescence and CPL. It offers a promising route for next-generation photonic materials that are metal-free, lightweight, and structurally tuneable.
In a study published in Communications Chemistry, the team showcased the potential of these new molecules in anti-counterfeiting. They formulated inks using the phosphorescent molecules and demonstrated how hidden information can be revealed selectively. For example, under UV light, a sample text message appears as ‘1180’ from its fluorescent response. But when the light source goes off, the text ‘IISc’ emerges in the dark, with a persistent afterglow enabled by the new molecule. This “time-gated” visibility makes such molecules ideal for security tags, encrypted labels, and tamper-proof authentication technologies.
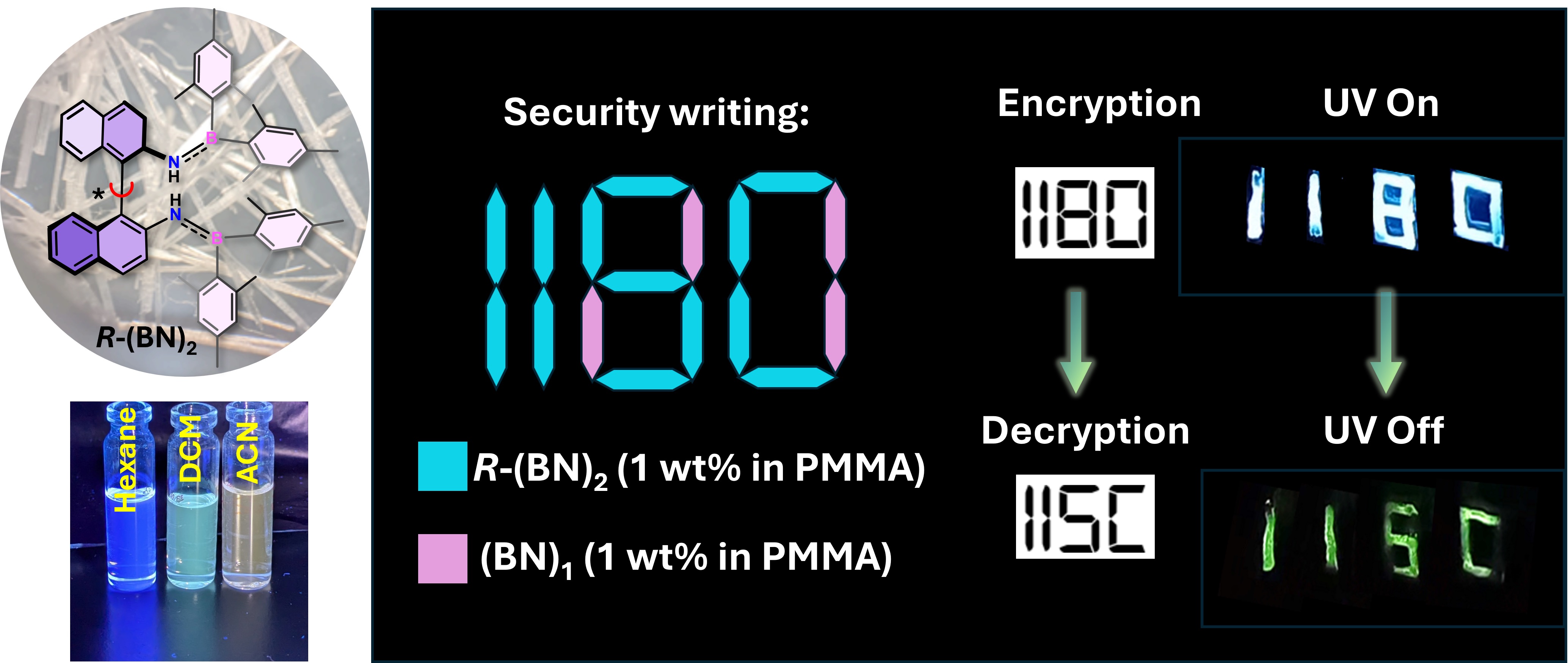
Left: Chemical structure of the chiral aminoborane along with its solvent-dependent luminescence behaviour. Right: Demonstration of security writing using ink formulated from 1 wt% R-(BN)₂ and its achiral analogue (BN)₁ in polymethyl methacrylate (PMMA). Both samples exhibit similar fluorescence under UV light, however, the chiral framework in R-(BN)₂ results in brighter phosphorescence and a prolonged afterglow at 298 K under a nitrogen atmosphere (Image: Jusaina Eyyathiyil)
When we shine light on a molecule in the stable ‘ground’ state, it gains energy and becomes excited. It can lose this energy by moving or hitting other molecules, without emitting light. Alternatively, it can return to the ground state quickly by giving off light in a process called fluorescence. Sometimes, through a rare phenomenon called ‘intersystem crossing,’ the molecule shifts from a singlet state, in which its electron spins are paired, to a triplet state, in which electron spins are unpaired and at a higher energy, before emitting light and returning to the ground state. Since this shift involves a change in spin, it is slower, and the light emitted lasts longer. This type of emission is called phosphorescence.
Phosphorescence is hard to achieve at room temperature because it involves electrons changing their spin state as well as significant energy loss due to molecular motion, which demands cryogenic conditions to observe phosphorescence with the naked eye. To address this, the IISc team developed a boron and nitrogen-containing organic molecule, known as an aminoborane, with a carefully engineered rigid structure. By locking two naphthalene-based chromophores around a central axis, the researchers were able to suppress non-radiative decay and enable long-lived phosphorescence.
“The B–N bond has the same structural properties as a C–C bond, but it blesses the molecule with excited states having different symmetries,” explains P Thilagar, Professor at IPC and corresponding author. “This enhances spin flipping and promotes intersystem crossing, enabling efficient phosphorescence at room temperature.”
The rigid chiral scaffold not only facilitated long-lived emission but also enabled circularly polarised luminescence – a rare and highly desirable feature in organic materials. “CPL is one of the most intriguing aspects of this molecule,” adds Jusaina Eyyathiyil, PhD student at IPC and first author of the study. “It’s a difficult phenomenon to observe in organic systems due to their typically low emission efficiency. However, with support from our collaborators, we were able to achieve it.”
Synthesising the molecule was no easy feat. “The formation of the B–N bond is highly sensitive to moisture and oxygen, and required strictly controlled conditions,” explains Eyyathiyil. “We carried out the reaction using n-butyllithium – a flammable reagent – under an inert atmosphere in a -78°C bath.” Crystallising the molecule to analyse its structure was also challenging. The team collaborated with the group of Neal Hickey at the University of Trieste, Italy, to access synchrotron facilities for crystal structure confirmation.
Looking ahead, the team aims to improve the emission efficiency of the system, which could enhance its utility in bioimaging and photonic materials. “With precise molecular design, we’re beginning to bridge the gap between structural rigidity and multi-functional emission,” says Thilagar. “This work adds a new chapter to the ongoing story of small organic phosphors.”

Members of P Thilagar group. Clockwise from top left: Jusaina Eyyathiyil, Manvi Sharma, Gayathri L, P Thilagar, and Ranjithkumar K (Photos courtesy: PT Lab)
REFERENCE:
Eyyathiyil J, Ghosh S, Cheran A, Geremia S, Kumar J, Hickey N, Thilagar P, Axial chirality-induced rigidification in aminoboranes enhances persistent room-temperature phosphorescence and circularly polarized luminescence, Communications Chemistry (2025).
https://www.nature.com/articles/s42004-025-01529-8
CONTACT:
P Thilagar
Professor
Department of Inorganic and Physical Chemistry (IPC)
Indian Institute of Science (IISc)
Email: thilagar@iisc.ac.in
Phone: +91 80 2293-3353
Website: https://sites.google.com/view/ptlab-ipc-iisc
NOTE TO JOURNALISTS:
a) If any of the text in this release is reproduced verbatim, please credit the IISc press release.
b) For any queries about IISc press releases, please write to news@iisc.ac.in or pro@iisc.ac.in.



