Unravelling an adaptation process of the tuberculosis pathogen
Mycobacterium tuberculosis, the bacterium that causes tuberculosis (TB), possesses the brilliant ability to sense threats and adapt inside the host. Its sensing and adaptive behaviour are primarily driven by signalling apparatuses known as two-component signalling systems (TCSs). A conventional TCS system comprises a receptor sensor kinase (SK) that perceives environmental fluctuations (changes in the signal strength) and a partner response regulator (RR) that receives the information from the SK and generates output. The TCSs have emerged as an attractive drug target because of their importance for bacterial survival.
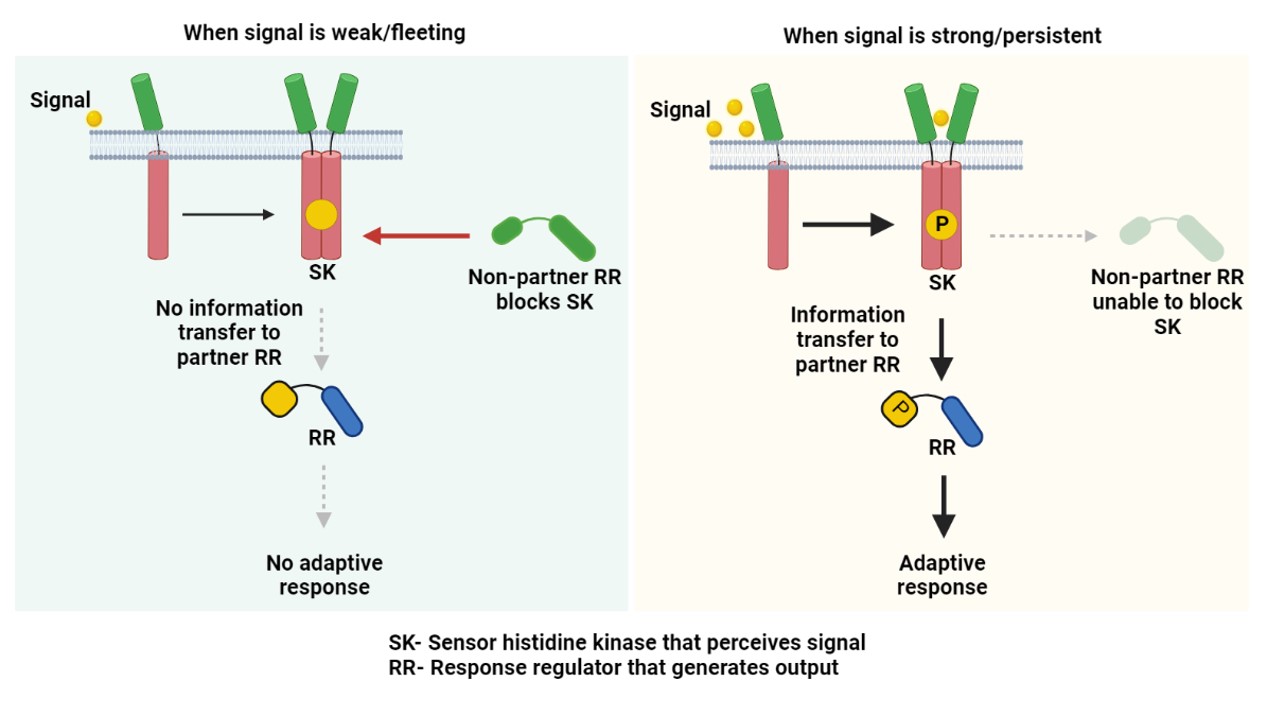
In a new study from the labs of Deepak K Saini (Department of Developmental Biology and Genetics) and Narendra M Dixit (Department of Chemical Engineering), the researchers have unravelled a mechanism that allows the TB bacterium to ignore small environmental stimulations it senses through these TCSs, thereby helping the bacterium avoid efforts to mount unnecessary and futile responses and conserve its energy. The bacterium has developed a resource-effective way of doing this. Stimulation of SK leads to direct physical communication with its partner RR (cognate TCS pair) and triggers an adaptive response. However, if this signal is weak or fleeting, another non-partner RR can bind and block (sequester) the SK, thereby not allowing it to communicate with its partner RR (Figure, left panel). On the other hand, when the signal is strong or persistent, the SK can override this non-partner barrier and generate an adaptive response to the signal. This mechanism sets a signal response threshold, below which all incoming signals are ignored, and a response is mounted only when the threshold is crossed.
Such sequestration or blocking of signalling by a non-partner is a simple design principle that helps delineate signal from noise. “Targeting such an interaction node can disarm the bacterium, and even weak stresses posed by immunity or drug regimens would generate a strong undesirable response in bacterium,” says Deepak, the corresponding author of the study. “Exploiting this finding to pin a sequestration interaction, in principle, can exhaust the pathogen, thereby increasing their susceptibility to immune pressures,” adds Gaurav Sankhe, one of the lead authors of the study. The study elegantly blends experimental and mathematical approaches used in the Saini and Dixit laboratories to extract intricate signaling processes from one of the most dreaded pathogens of all time.
REFERENCE:
Sankhe GD, Raja R, Singh DP, Bheemireddy S, Rana S, Athira PJ, Dixit NM, Saini DK, Sequestration of histidine kinases by non-cognate response regulators establishes a threshold level of stimulation for bacterial two-component signalling, Nature Communications (2023).
LAB WEBSITES:



