3D bioprinted peripheral nerve conduits
– Sankeerthana Avasarala (with input from authors)
Peripheral nerve damage can have debilitating consequences, ranging from intense and persistent pain to complete loss of sensation in the affected area. The peripheral nervous system, consisting of 43 nerve pairs, serves as a vital communication network connecting the central nervous system (CNS), which comprises the brain and spinal cord, to the entire human body. It plays a crucial role in controlling movement, facilitating our sense of touch, and coordinating various everyday activities. Severe nerve injuries often result in damage to the axon, the component of a nerve cell responsible for transmitting signals to the central nervous system and surrounding tissue. This damage can significantly impair sensory and motor functions, leading to profound physical and functional limitations for affected individuals.
Traditional approaches, such as autografts and allografts, have limitations in terms of improper fitting, loss of communication with the CNS, donor site morbidity, donor-host mismatch, and host immune response. These challenges collectively impede clinically acceptable functional recovery, especially for critical nerve gaps (>10 mm).
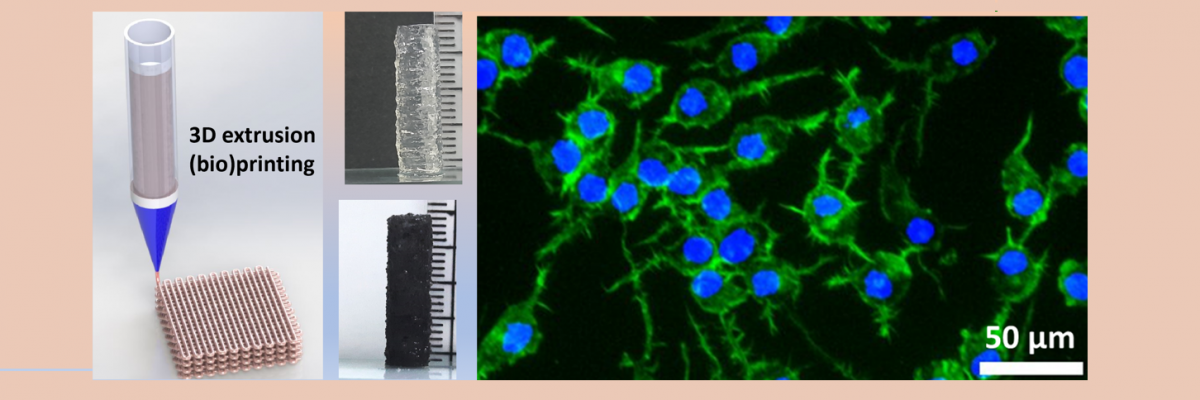
A nerve guidance conduit (NGC) is a medical device designed to function as an artificial nerve graft, aiding in the process of axonal regrowth and nerve regeneration by bridging the gaps in damaged nerves, providing physical support, guidance, and a conducive microenvironment for nerve fibers to grow and reconnect. Recently developed cutting-edge 3D bioprinting technology enables precise control over the deposition of cells, biomaterials, and bioactive molecules, allowing for the creation of intricate structures of NGC that closely mimic natural tissues.
Despite substantial progress in recent decades, the aspiration to create fully functional cellularised artificial NGCs through 3D bioprinting remains abortive for clinical application. The key challenge stems from our inability to build critical-sized nerve conduit structures mimicking the intricate properties of native functional tissue. This demands the formulation of an ideal bioink, comprising living cell suspensions within a biomaterial matrix, which must exhibit excellent printability and furnish a tissue-specific microenvironment conducive to neural cell growth and differentiation.
In a new study, researchers at the Materials Research Centre, Indian Institute of Science (IISc), led by Bikramjit Basu, have introduced a novel bioink for the development of functional NGCs. This innovative bioink comprises photocrosslinkable gelatin methacryloyl as the primary matrix material, poly(ethylene glycol) diacrylate as the regulator of mechanical properties, gellan gum as the rheological modifier, and carbon nanofibers as the electroactive nanoparticle.
For fabrication of the NGCs, the research team collaborated with Avay Biosciences Private Limited, India, in line with the ‘Start-up India’ initiative. Together, they developed a customised 3D extrusion bioprinter tailored to their specific requirements. This fully customised printer, now successfully commercialised, has garnered significant acclaim within the indigenous biofabrication field for its exceptional performance. Leveraging the capabilities of the nozzle-assisted 3D extrusion printer, the IISc researchers successfully constructed NGCs with varying inner diameters ranging from 4 to 10 mm and a length of 20 mm. Remarkably, these NGCs exhibited excellent printability and buildability using the multifunctional electroactive biomaterial inks. In establishing 3D bioprintability, the researchers incorporated live nerve cells into the fabricated NGCs, confirming the cytocompatibility of the hydrogels.
This study underscores the potential of these NGCs to mimic the natural tissue environment, thus paving the way for significant advancements in NGC development and nerve regeneration therapies. The research group is presently engaged in a preclinical study aimed at assessing the efficacy of these conduits in promoting nerve growth in mice. This study involves the application of external electrical stimuli to augment the regenerative potential of the conduits.
REFERENCE:
Das S, Jegadeesan JT, Basu B, Advancing Peripheral Nerve Regeneration: 3D Bioprinting of GelMA-Based Cell-Laden Electroactive Bioinks for Nerve Conduits, ACS Biomaterials Science and Engineering (2024). https://doi.org/10.1021/acsbiomaterials.3c01226
LAB WEBSITE:
https://bikramjitbasu.in/
GROUP PHOTO:




