A new spectroscopy technique for aqueous solutions
Measuring proteins, lipids, collagen, and sugars is essential in biomedical research, for diagnostics or therapeutics. Knowing the concentration of these molecules can help us differentiate healthy from diseased tissues. Infrared (IR) optoacoustic spectroscopy can separate a multitude of molecules based on their absorption spectra. However, IR optoacoustic spectroscopy is limited when measuring target molecules in aqueous solution due to the strong water absorption at IR wavelengths, which reduces the detection sensitivity. In such cases, Raman spectroscopy is considered, as it detects symmetric vibrations of nonpolar molecules and is therefore insensitive to water absorption. Raman scattering by biological samples generally leads to low signal-to-noise ratio for detailed studies, reflecting the fact that usually only 1 in 1010 photons undergo a Stokes or anti-Stokes shift.
Based on the dependence of the optoacoustic signal on the temperature of the probed medium, a cooled IR optoacoustic spectroscopy (CIROAS) approach was developed to mute water contributions in optoacoustic spectroscopy. The study, carried out by Jaya Prakash, Assistant Professor in the Department of Instrumentation and Applied Physics, at Prof. Ntziachristos’ group in TUM, shows that spectral measurements of proteins, lipids, and glucose at the short-wavelength IR region, performed at 4°C, lead to marked sensitivity improvements over conventional optoacoustic or IR spectroscopy. The researchers elaborate on the dependence of optoacoustic signals on water temperature and demonstrate polarity changes in the recorded signal at temperatures below 4°C. They also elucidate the dependence of the optoacoustic signal and the muting temperature on sample concentration, and demonstrate that changes in these dependences enable quantification of the solute concentration. The study also discusses how CIROAS may enhance abilities for molecular sensing at IR wavelengths, and the possibility of CIROAS being used for conventional laboratory measurements or for analysing biological fluids such as blood.
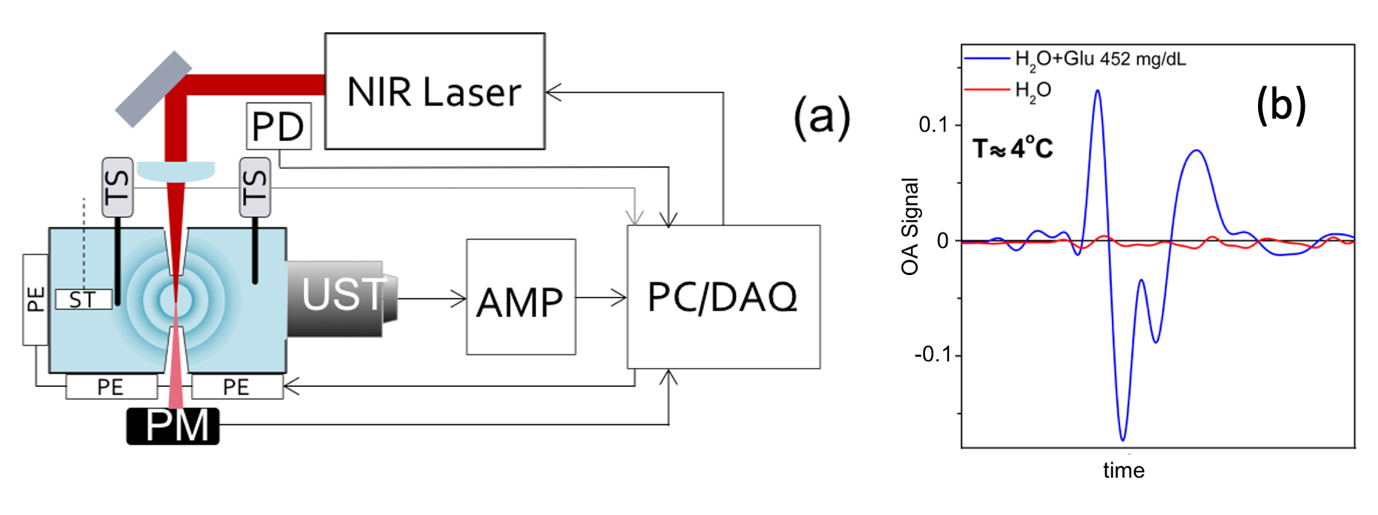
Fig. 1: (a) Schematic representation of the experimental setup for optoacoustic spectroscopy. UST, ultrasound transducer; PD, photodiode; AMP, amplifier; TS, temperature sensor; PM, power meter; ST, stirrer; PE, Peltier element; PC/DAQ, personal computer with data acquisition card. (b) Superposition of the optoacoustic signals of pure water (red line) and the aqueous solution of glucose (blue line) at 4°C. Fig. adapted from below indicated reference.
REFERENCE:
Jaya Prakash*, Mir Mehdi Seyedebrahimi*, Ara Ghazaryan*, Jaber Malekzadeh-Najafabadi, Vipul Gujrati, and Vasilis Ntziachristos, “Short wavelength optoacoustic spectroscopy based on water muting,” Proceedings of the National Academy of Sciences 117, 4007-4014 (2020). (*Equal Contribution DOI: 10.1073/pnas.1910597117)
LAB WEBSITE:
https://sites.google.com/view/jayaprakash/home




