22 September 2025
– Ashmita Gupta
Every time a eukaryotic cell divides, it faces a monumental challenge: It must carefully duplicate and divide its genetic material (chromosomes) equally, and then rebuild the nuclear envelope around the separated halves. If this process goes wrong, the resulting nuclei can be misshapen or disorganised – features often seen in cancer and ageing-related diseases. A new study from researchers at the Indian Institute of Science (IISc) and Université Paris-Saclay reveals how a key enzyme called Aurora A helps cells pull off this feat.
In dividing cells, structures called spindle poles (or centrosomes) grow in size to generate the microtubule ‘tracks’ that pull chromosomes apart. Once this job is done, the spindle poles must shrink and disassemble so that the nuclear envelope can reform around the separated chromosomes.
“When cells exit mitosis, the spindle poles disassemble. Why these structures have to disappear, what their material properties are, and why those are relevant – these are not known,” says Sachin Kotak, Associate Professor at the Department of Microbiology and Cell Biology (MCB), IISc, and corresponding author of the study.
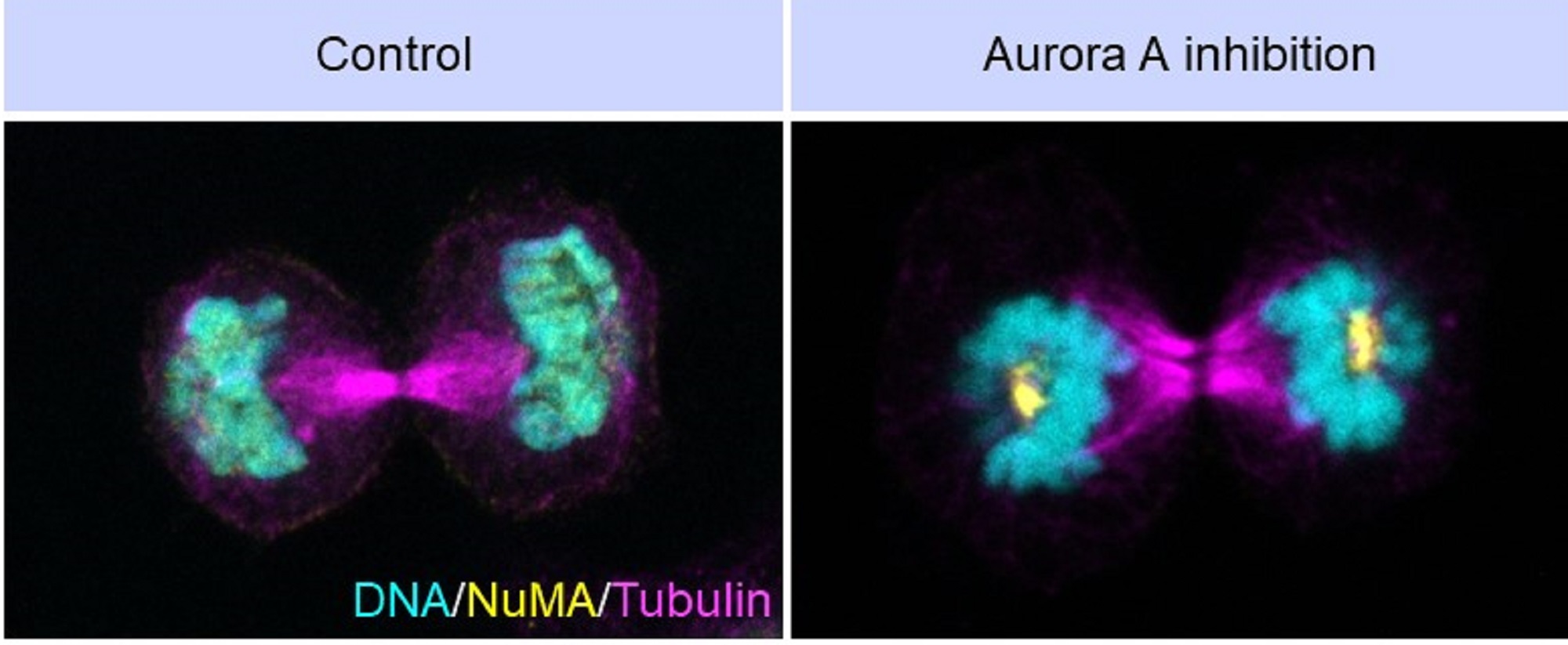
The team found that Aurora A plays a crucial role in ensuring that the spindle poles dissolve at the right time. Without this enzyme, the poles remain ‘sticky’, causing the chromosomes to bend awkwardly around them and leading to distorted nuclei.
Role of Aurora A kinase in proper chromosome segregation and nuclear assembly (Image: Sachin Kotak)
Another protein central to this process is NuMA, which is essential for spindle pole organisation. Normally, NuMA gathers at spindle poles during mitosis and then disperses as cells complete dividing. Aurora A keeps NuMA in a dynamic, liquid-like state, allowing it to move in and out of spindle poles.
When Aurora A is inhibited, however, NuMA hardens into a more solid-like state and clumps at the poles. Using advanced imaging techniques, the team showed how Aurora A–mediated phosphorylation of NuMA prevents the latter from hardening at the poles. The team also identified specific regions and amino acids in NuMA that drive this shift between dynamic and solid states.
“This work shows that it’s not just about which proteins are present, but also about their material state – whether they behave like liquids or solids,” explains Kotak. “Aurora A ensures that spindle poles dissolve smoothly, allowing the genome to be properly encased in new nuclei.”
A major challenge in studying Aurora A was that removing it too early halts mitosis, making it hard to probe the enzyme’s role in later stages.
“One of the cool things we have done in this work is make a tool by which we can destroy Aurora A when cells exit mitosis,” says Kotak. This allowed them to show that the enzyme’s later role in nuclei formation is distinct from its well-known earlier function in spindle assembly.
The findings highlight the elegant choreography involved in mitosis and open up the possibility of targeting these protein states for therapeutic applications.

Research team from right to left: Madhumitha Balakrishnan, Dwaipayan Chakrabarty, Vignesh Olakkal (co-first authors of the paper), and Sachin Kotak (Photo: Prajwal Datta)
REFERENCE:
Rajeevan A, Olakkal V, Balakrishnan M, Chakrabarty D, Charon F, Noordermeer D, Kotak S, Aurora A regulates the material property of spindle poles to orchestrate nuclear organization at mitotic exit, EMBO Journal (2025).
https://www.embopress.org/doi/full/10.1038/s44318-025-00564-4
CONTACT:
Sachin Kotak
Associate Professor
Department of Microbiology and Cell Biology (MCB)
Indian Institute of Science (IISc)
Email: sachinkotak@iisc.ac.in
Phone: +91-80-23607717; +91-80-22932292
Website: https://kotakcellbiology.wixsite.com/spindlebehaviour
NOTE TO JOURNALISTS:
a) If any of the text in this release is reproduced verbatim, please credit the IISc press release.
b) For any queries about IISc press releases, please write to news@iisc.ac.in or pro@iisc.ac.in.



