25 October 2023
– Sukriti Kapoor
An interdisciplinary team of researchers from the Indian Institute of Science (IISc) has uncovered how the stiffness of a cell’s microenvironment influences its form and function. The team was led by Namrata Gundiah, Professor at the Department of Mechanical Engineering and Paturu Kondaiah, Professor at the Department of Developmental Biology and Genetics. The findings provide a better understanding of what happens to tissues during wound healing.
Inefficient wound healing results in tissue fibrosis, a process that can cause scar formation, and may even lead to conditions like cardiac arrest. Changes in the mechanical properties of tissues – like stiffness – also happen in diseases like cancer.
In the study, published in Bioengineering, the team cultured fibroblast cells – the building blocks of our body’s connective tissue – on a polymer substrate called PDMS with varying degrees of stiffness. They found that a change in the stiffness altered cell structure and function. Fibroblast cells are involved in extensive remodelling of the extracellular matrix (ECM) surrounding biological cells. The ECM, in turn, provides the mechanical tension that cells feel inside the body. The team found that fibroblasts cultured on substrates that had lower stiffness were rounder and showed accompanying changes in the levels of cytoskeleton proteins such as actin and tubulin. Moreover, fibroblasts grown on such substrates showed cell cycle arrest, lower rates of cell growth and cell death.
To pinpoint the “master regulator” that drives changes in the cell when substrate stiffness changes, the team focused their attention on an important signalling protein called Transforming Growth Factor-β (TGF-β). Previous work has shown that the activity of fibroblasts and the downstream ECM architecture is regulated by TGF-β. “The thing is, people talk about the chemical changes … but not about biomechanical,” says Brijesh Kumar Verma, former PhD student at the Department of Developmental Biology and Genetics, IISc, and first author of the study. For example, while the TGF-β signalling cascade has been studied extensively in cancer, the influence of mechanical forces – such as substrate stiffness – has not been studied so far, Verma adds.
The ECM surrounding different tissues has different levels of stiffness – from being soft around the muscle, to very hard around bone. To mimic this diversity, the team fabricated PDMS substrates of varying stiffness on which fibroblasts were grown. “You can use PDMS to create biocompatible materials with substrate stiffness over large orders of magnitude, from 40 kilopascals to more than 1.5 megapascals,” explains Aritra Chatterjee, former PhD student at the Department of Bioengineering, IISc, and another author.
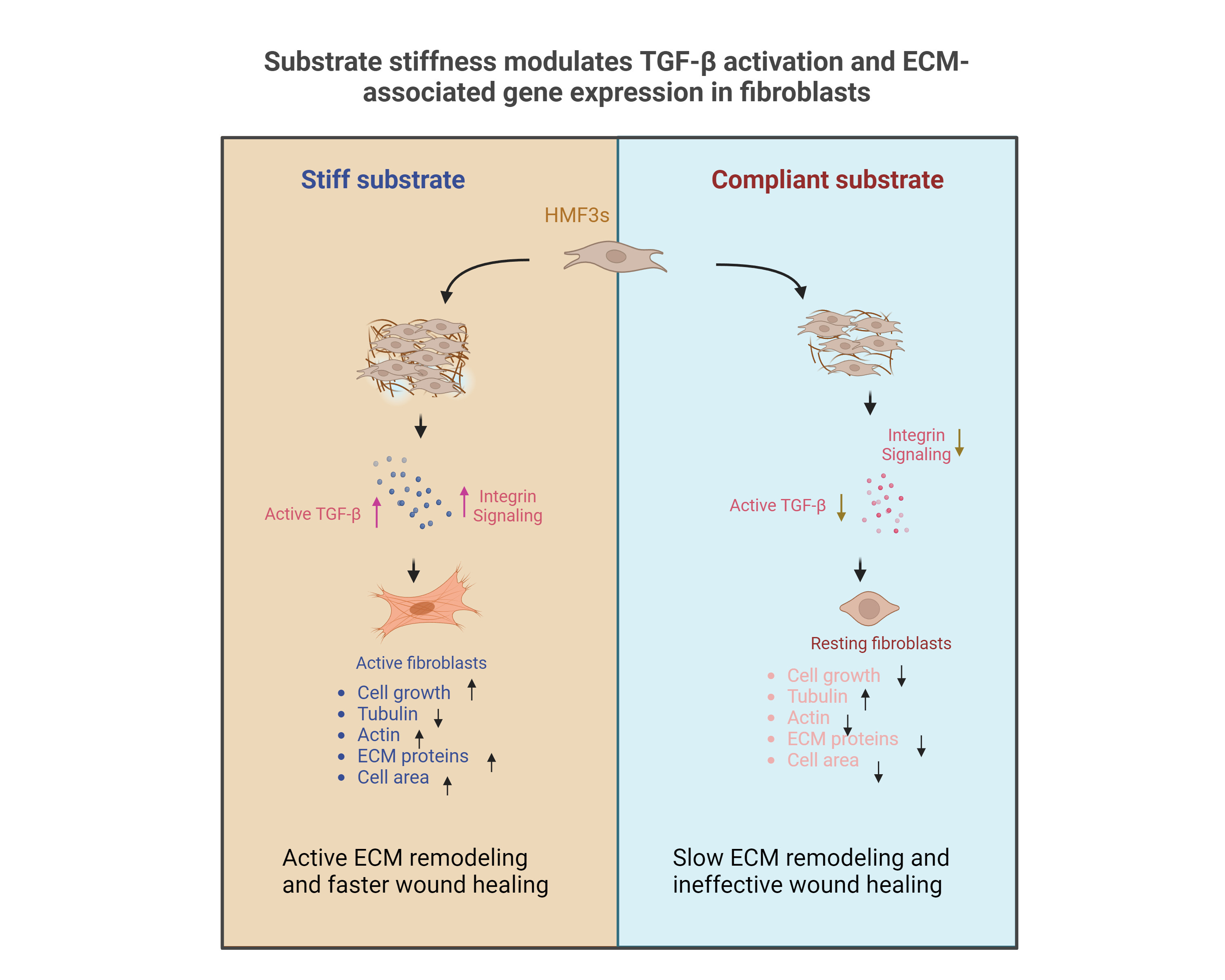
Image: Brijesh Kumar Verma and Aritra Chatterjee
At first, the researchers did not observe any changes in the total TGF-β levels. “[Interestingly], when we did the activity-based assay for TGF-β, we were quite surprised,” says Verma. They found that when substrate stiffness increased, TGF-β activity also increased – in other words, the levels of the active form of the protein started rising. Verma adds that this could explain why wound healing occurs at different rates in different tissues. This means that bone tissue, which grows on a stiffer ECM, may be less prone to scarring upon injury when compared to muscle tissues, which reside in a softer biomechanical environment.
The team also found that there was an uptick in the production of several ECM components when the substrate stiffness increased – fibroblasts growing on an already stiff substrate also start secreting more ECM components, in a positive feedback loop. “The most novel finding was the fact that the signalling [between the fibroblast and ECM] was actually sensitive to a mechanical stimulus, which is substrate stiffness,” Chatterjee explains.
In the future, the researchers seek to understand how other mechanical factors, such as surface properties and cell stretch, can also influence TGF-β activity.
“The microenvironment of the cell is very complicated as it is experiencing a lot of different forces,” says Chatterjee. Understanding their influences and tracking the biophysical parameters of the cell can also provide a useful tool to distinguish between healthy and cancer cells. A tumour mass can be targeted more efficiently if we understand how stiffness changes in diseased cells, Verma explains. “I’m very optimistic about this.”
REFERENCE:
Verma BK, Chatterjee A, Kondaiah P, Gundiah N, Substrate Stiffness Modulates TGF-β Activation and ECM-Associated Gene Expression in Fibroblasts, Bioengineering (2023)
CONTACT:
Brijesh Kumar Verma
Postdoctoral Research Scholar
University of Pennsylvania, Philadelphia
Former PhD student, Department of Developmental Biology and Genetics, Indian Institute of Science (IISc)
Email: brijeshkumar.verma@pennmed.upenn.edu
Aritra Chatterjee
Assistant Professor
Department of Mechanical Engineering
Birla Institute of Technology & Science, Pilani, Hyderabad
Former PhD student, Department of Bioengineering, Indian Institute of Science (IISc)
Email: aritra.chatterjee@hyderabad.bits-pilani.ac.in
Namrata Gundiah
Professor
Department of Mechanical Engineering
Indian Institute of Science (IISc)
Email: namrata@iisc.ac.in
Phone: +91-80-22932860
Paturu Kondaiah
Former Professor and Chair
Department of Developmental Biology and Genetics
Indian Institute of Science (IISc)
Email: paturu@iisc.ac.in
Phone: +91-80-22933259
NOTE TO JOURNALISTS:
a) If any of the text in this release is reproduced verbatim, please credit the IISc press release.
b) For any queries about IISc press releases, please write to news@iisc.ac.in or pro@iisc.ac.in.

