Frog skin peptide blocks enzymes of disease-causing pathogens
– Shloak Vatsal
We encounter an array of pathogens (disease-causing agents) in our daily lives adept at evading our immune system. And many of these produce enzymes that block protective proteins in our body. Hence, it is imperative to devise effective counterstrategies to tackle them. Researchers at IISc have identified a potent molecule, derived from amphibians, with the ability to combat such pathogens.
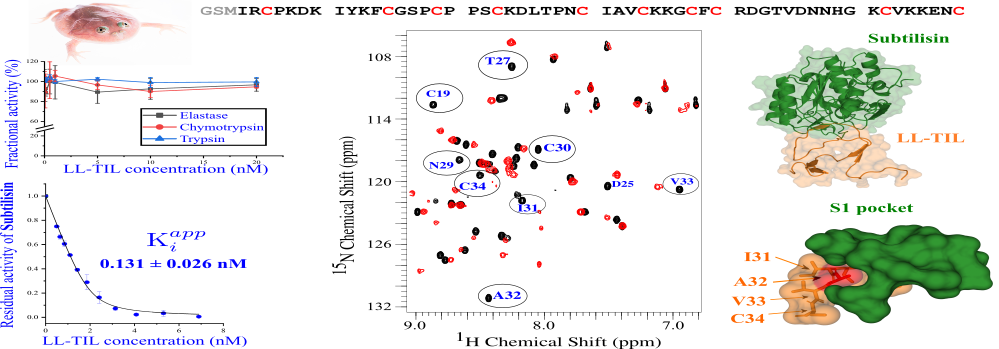
Peptides (short proteins) produced from the skin of amphibians have long been studied because of their ability to counter unfavourable conditions in the environment, including harmful pathogens. The IISc team, comprising Mihir Rami, Mohd Shafique and Siddhartha Sarma at the Molecular Biophysics Unit, has studied LL-TIL, one such peptide found in skin secretions of Budgett’s Frog. It is a large aquatic frog found in South America, made popular by memes for its comical rounded appearance.
The researchers found that the frog-secreted peptide inhibited two key enzymes called subtilisin Carlsberg and proteinase K, produced by pathogens. These enzymes play a pivotal role in promoting infections by degrading specific protective proteins of the infected person. The team used various spectroscopic techniques and protein assays to study the binding of the amphibian peptide to the pathogenic enzymes. The peptide was shown to act through a ‘slow-tight binding’ pathway, and was found to be as effective as SSI, a well-known subtilisin inhibitor.
The study further revealed an in-depth mechanism of this inhibitory action, using structural and dynamic models. The researchers show the formation of a Michaelis complex – a tight, noncovalent complex with the intact inhibitor – during the process. They also studied the effects of modifications to the original sequence of the peptide. This provides a framework to engineer more specific and potent TIL-type inhibitors, which can be used against other pathogenic enzymes as well.
As diverse pathogens secrete enzymes similar to subtilisin and proteinase K, the ability of LL-TIL to inhibit them makes it a potentially valuable molecule.
REFERENCE:
Rami M, Shafique M, Sarma SP, Structural, Functional, and Mutational Studies of a Potent Subtilisin Inhibitor from Budgett’s Frog, Lepidobatrachus laevis, Biochemistry (2023).
https://doi.org/10.1021/acs.biochem.3c00252
LAB WEBSITE:
http://mbu.iisc.ac.in/~siddlab/public_html/research.html



